 |
Plant Physiology (Biology 327) - Dr. Stephen G. Saupe; College of St. Benedict/ St. John's University; Biology Department; Collegeville, MN 56321; (320) 363 - 2782; (320) 363 - 3202, fax; ssaupe@csbsju.edu |
 |
Plant Physiology (Biology 327) - Dr. Stephen G. Saupe; College of St. Benedict/ St. John's University; Biology Department; Collegeville, MN 56321; (320) 363 - 2782; (320) 363 - 3202, fax; ssaupe@csbsju.edu |
Introduction to the Protoplast Lab
Objectives: Upon completion of this set of labs you should be able to:
A Protoplast Primer:
Protoplasts, plant cells without walls, are important tools in the
study of plant physiological activities. They have been used to: (1) separate cell types;
(2) study the intracellular location of particular metabolites; (3) gently isolate
organelles from plant cells; (4) study cell wall and plasma membrane biosynthesis; (5)
produce somatic cell hybrids (fusion of protoplasts); (6) introduce foreign genetic
material and organelles into recipient plants; and (7) achieve highly efficient virus
infections (Wagner et. al., 1978).
Protoplasts are most easily isolated by enzymatically digesting the cell wall. The general procedure is: (1) surface sterilize the leaf; (2) rinse the tissue in the proper osmotic agent such as sorbitol or mannitol. Protoplasts must be maintained in an isotonic medium so they don't burst; (3) cut the leaf into strips or peel the epidermis to expose the tissue for enzymatic digestion; (4) treat with enzymes (sequentially or mixed). Typically, a combination of cellulase, pectinase and hemicellulase, is used. The specific amount and type of enzyme used is generally a result of trial and error. Cellulysin and Meicelase are trade names for cellulase and pectinase, respectively; (5) rinse the preparation to remove the enzymes; and (6) isolate and purify the protoplasts. An alternative method to isolate protoplasts is to mechanically chop up the plant tissue in isotonic medium (McLaughlin, Giannini, Fishbeck, 1993).
Protoplasts can be ruptured in a variety of ways to release the cell contents. Three commonly used methods include: (1) mechanical shear; (2) osmotic shock; and (3) mild detergent. Each method has specific advantages and disadvantages and the method selected depends upon the application. For example, forcing protoplasts through a syringe or fine nylon mesh (~ 20 μm) will rupture protoplasts including the vacuoles. Osmotic shock will gently disrupt the protoplast releasing intact vacuoles. Treating protoplasts with K2HPO4 causes the cytoplasm to congeal and release vacuoles.
An Anthocyanin Primer:
Anthocyanins, which are a type of flavanoid pigment, are
responsible for the coloration of red cabbage leaves. These pigments are water soluble and
are dissolved in the cell sap in the vacuole. Thus, they are a good marker to observe the size
of the vacuole. In cabbage, anthocyanins may be synthesized in anthocyanoplasts which are
tiny, transient, organelle-like structures found in the vacuole (look for discrete red
spots or speckles). The concentration of anthocyanin in the vacuole of
grape skin cells is 97 mM (Moskowitz and Hrazdina, 1981).
Anthocyanins are polyphenolic (see structure), water-soluble molecules responsible for the color of many flowers and fruits. There are 15 different anthocyanins in red cabbage. Most have the basic cyanidin skeleton to which are attached various groups including glucose, coumarin, ferulic acid, and sinapic acids. The basic anthocyanin skeleton is drawn below:
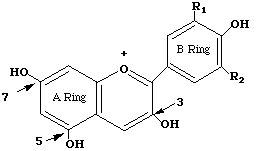 Anthocyanins are pH sensitive. The color of an individual
anthocyanin will vary from red (acidic conditions) to blue to yellow (alkaline
conditions). The final tissue color is ultimately dependent upon several factors including
the type and concentration of anthocyanin pigments present, pH, and the presence of other
metabolites (such as ions, sugars, hormones).
Anthocyanins are pH sensitive. The color of an individual
anthocyanin will vary from red (acidic conditions) to blue to yellow (alkaline
conditions). The final tissue color is ultimately dependent upon several factors including
the type and concentration of anthocyanin pigments present, pH, and the presence of other
metabolites (such as ions, sugars, hormones).
Anthocyanin synthesis requires light. Low levels of anthocyanin are present in dark grown plants (0.35 nmol/g) but the amount increases rapidly after exposure to light (5 nmol/g after 6 days). IAA treatment decreases anthocyanin synthesis and K+ stimulates it (Mazza & Minati, 1993).
Overview:
In this lab, we will isolate
and purify protoplasts from red cabbage and examine them in the
microscope. If time we will examine the properties
and the viability of the
protoplasts. We will spectrophotometrically determine the anthocyanin
content of the red cabbage protoplasts. To accomplish this task we will
also need to
determine the protoplast volume with an
ocular micrometer and quantify protoplast numbers in our
preparation using a hemocytometer.
Pre-Lab: Print and complete the pre-lab questions.
Post-Lab: At the completion of the lab experience you are required to complete:
- Sketch or print of digital imags of the red cabbage and green leaf (species to be determined) protoplasts we observed including an appropriate caption. Label the visible structures. Include plate magnification. Label your images (e.g., Figure 1, 2) giving each an appropriate caption.
- Tables 1 & 2 summarizing protoplast yield data. Are the data significant at the 0.05 confidence level?
- Table 1 for the frequency of pigmented and unpigmented protoplasts
- Table 2 & 3 summarizing volume data for pigmented and unpigmented protoplasts
- On a separate sheet of paper show a statistical analysis to answer the question, "Are pigmentd protoplasts larger than unpigmented protoplasts?" Record Ho, t value from t-test, p value and your conclusion about the hypothesis/question.
- Tables 1 & 2 summarizing anthocyanin data
References:
| | Top | SGS Home | CSB/SJU Home | Biology Dept | Biol 327 Home | Disclaimer | |
Last updated:
01/07/2009 � Copyright by SG
Saupe