CO25. Summary of Elementary Steps
The reactions of carbonyls can become very complicated, involving many steps. In essence, though, the steps involve only a few, different elementary reactions.
-
A lone pair is donated from a nucleophile to the carbonyl carbon, forming a bond.
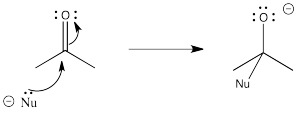
Figure CO25.1. Donation to a carbonyl.
-
Protons are most often transferred from a positively charged atom to a neutral atom with a lone pair.
-
Protons are also easily transferred from a positively charged atom to a negatively charged atom.
-
Sometimes, a protos might be transfered from a neutral atom to a negatively charged one.
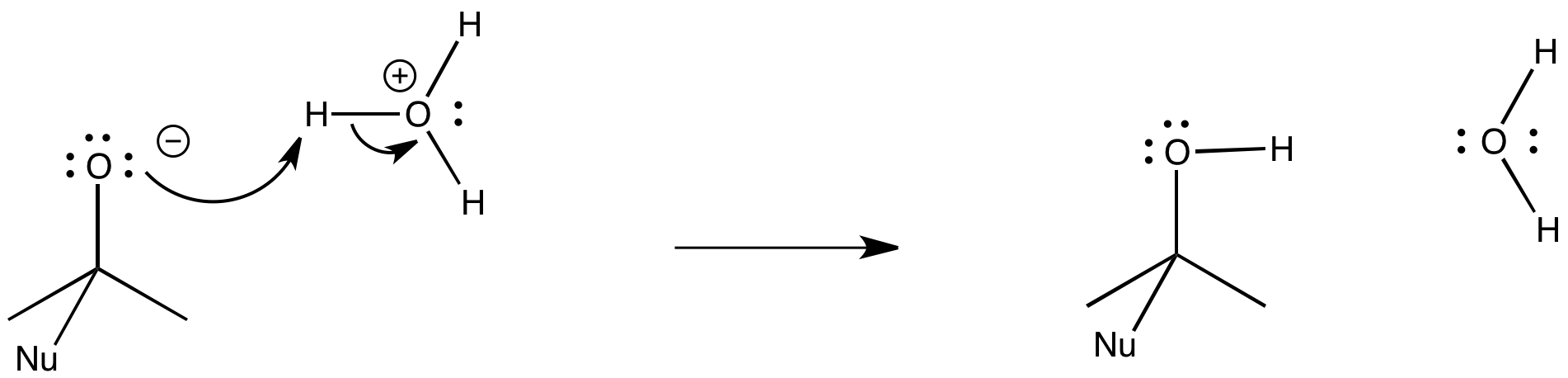
Figure CO25.2. Proton transfer.
In many of the reactions of anionic and semi-anionic nucleophiles, these two steps complete the entire reaction mechanism. However, if an additional lone pair can be revealed at the nucleophilic atom (often by transferring a proron away from this site), additional steps occur.
-
In pi donation, two heteroatoms, both with lone pairs, are attached to the same carbon. A lone pair is donated to the carbon, and one of the heteroatoms is pushed off.
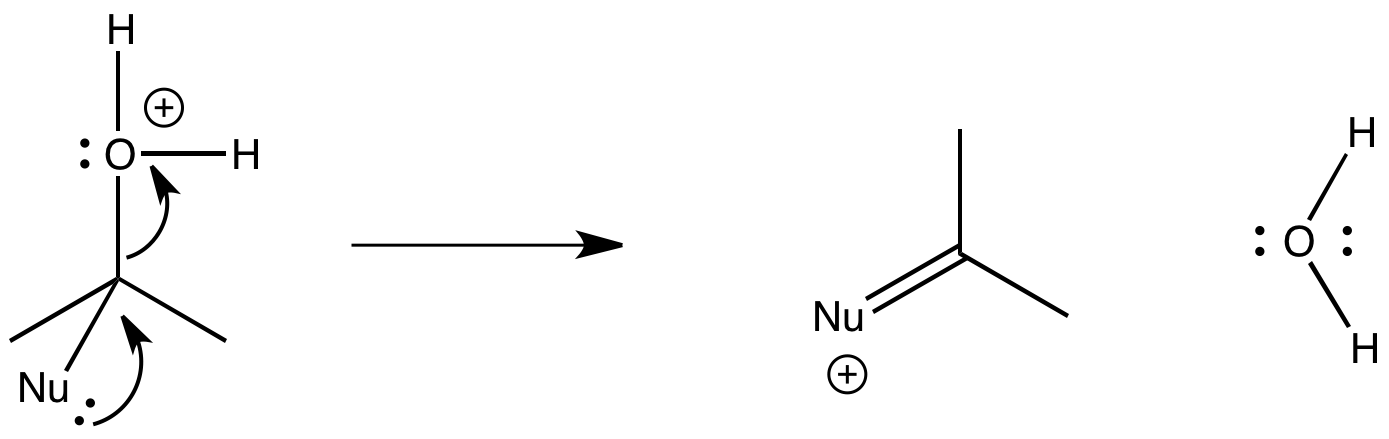
Figure CO25.3. π-Donation.
Many mechanisms involve a number of proton transfers and pi donations. These steps occur over and over, inching the molecule along step by step towards the product. Usually, each proton transfer helps to prepare an atom for eventual removal via pi donation.
Occasionally, if the nucleophile is neutral, these steps are preceded by an initial activation step.
-
Usually makes the reaction faster.
-
Is especially helpful when the nucleophile is uncharged, and hence less reactive.
-
The carbonyl is often activated by a proton (from a protic acid) but it can also be activated by a Lewis acid (such as a metal ion).

Figure CO25.4. Activation of a carbonyl.
