Reactivity in Chemistry
Radical Reactions
RR8. Radical Addition
Radical addition to alkenes is another classic example of a radical reaction. Like radical substitution, it illustrates some important elements of radical reactivity.
The most common example of radical addition to alkenes seen in college chemistry textbooks is radical addition of hydrogen bromide, HBr. That's because it complements the usual addition of HBr to an alkene.
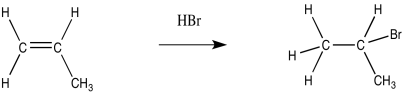
In the usual addition, HBr adds in a Markovnikov fashion to place the bromine at the more-substituted end of the alkene and the hydrogen on the less substituted end (remember the adage, "the rich get richer and the poor get poorer").

Figure RR8.1. A regular "Markovnikov" addition of HBr to an alkene.
In contrast, addition of HBr under radical conditions leads to the bromine attaching at the least-substituted position, whereas the hydrogen bonds to the most-substituted position. This is a Robin Hood reaction.
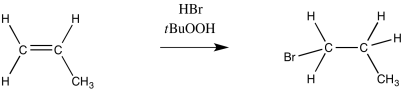
Figure RR8.1. An "anti-Markovnikov" addition of HBr to an alkene.
How does that reversal in regiochemistry come about? Consider the mechanism of the reaction.
Radical addition of HBr is almost always done in the presence of peroxides. The peroxide acts as an initiator for the reaction. The O-O bond may break through the simple action of thermal energy (maybe even at room temperature).
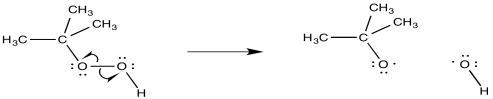
Figure RR8.3. Radical initiation from a peroxide.
The alkoxy or hydroxy radicals that result from this initiation step are left to induce radical propagation. Certainly one of the easiest available targets is the relatively weak H-Br bond. Oxygen radicals are very effient at hydrogen atom abstraction because of the strong O-H bond that results (approximately 120 kcal mol-1). In fact, oxygen radicals are frequently observed to abstract hydrogen atoms rather than adding to π bonds. Abstraction of a hydrogen atom from HBr produces a bromine radical.
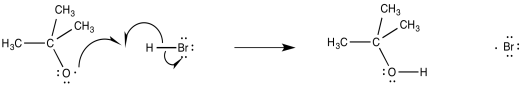
Figure RR8.4. Propagation via hydrogen atom abstraction by an alkoxy radical.
One of the things that the bromine radical could do is add to the double bond of an alkene. When it does so, it will bind to one end or the other of the former double bond. To which end will it go?
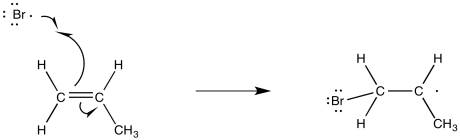
Figure RR8.5. Propagation via π-bond addition of bromine radical to an alkene.
Like cations, radicals are considered to be somewhat electron-deficient. They are stabilized by electron-donating factors. That means that, like a cation, the radical will be more favourable on the most-substituted carbon of the formal double bond.
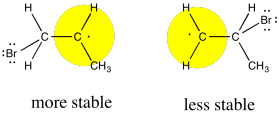
Figure RR8.6. The bromine radical adds to the less-substituted end of the alkene because the resulting is more stable at the more-substituted end.
Notice that this step is actually governed by almost the exact same factor that governs the polar addition of HBr. The regiochemistry is governed by the stability of the intermediate. Once that event has transpired, the regiochemistry of the product is fixed. It only remains for the alkyl radical to pluck a hydrogen atom from another HBr molecule, forming the final product and generating another bromine radical.
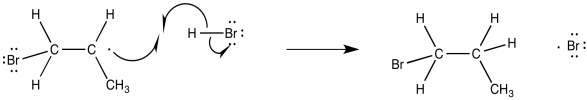
Figure RR8.7. The final step in alkyl bromide production also propagates another bromine radical.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: