Reactivity in Chemistry
Reaction Kinetics
RK5. Free Energy of Activation
There is really more to the activation energy than we have seen so far. The activation free energy is constant for a given reaction at a given temperature. But at different temperatures, ΔG changes. Just as in thermodynamics, it can be broken down in turn to:
ΔG = Δ H - TΔS
in which ΔH = activation enthalpy and ΔS = activation entropy.
The activation enthalpy is the part that corresponds most closely with the energy required for the reaction, the way we have been describing the activation barrier so far.
The activation entropy deals with how the energy within the molecule must be redistributed for the reaction to occur. One of the major factors influencing energy distribution over the course of the reaction is molecular geometry.
For example, suppose two molecules need to come together for a reaction to take place. They need to collide with each other. However, a reaction might not happen each time the molecules collide. Sometimes, the molecules may be pointing the wrong way when they bump into each other, so that the reaction can't occur. Often, atoms need to be lined up in the proper place where they will be forming a new bond.
When molecules are restricted to only certain orientations or geometries, they have fewer degrees of freedom. With fewer degrees of freedom, energy can be stored in fewer ways. As a result, there is often an entropy cost in initiating a reaction.
On the other hand, a reaction might start in a very different way, with one molecule breaking a bond and dividing into two pieces. Because each individual piece can move independently from the other, the degrees of freedom increase. Energy can be stored in more ways than they could be before the reaction started. As a result, although this reaction would still have an activation barrier, the entropy component may actually lower that barrier a little bit.
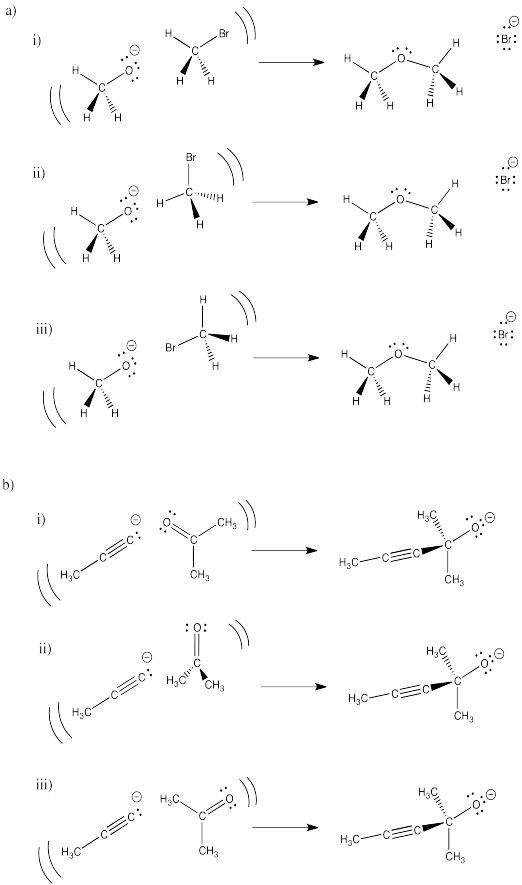
Problem RK5.1.
In the following drawings, one orientation of reactants is more likely to lead to the product shown. Select which one will be most successful in each set and explain what is wrong with each of the others.

Problem RK5.2.
Because the activation barrier depends partly on the energy needed to break bonds as the molecule heads into the transition state, comparative bond strength can be a useful factor in getting a qualitative feel for relative activation barriers.
The metal-carbonyl (M-CO) bond strengths of the coordination complexes M(CO)6 have been estimated via photoacoustic calorimetry and are listed below, by metal.
Cr: 27 kcal/mol Mo: 32 kcal/mol W: 33 kcal/mol
a) Based on that information, sketch qualitative activation barriers for the loss of a CO ligand from Cr(CO)6, Mo(CO)6 and W(CO)6.
b) Predict the relative rates for these three reactions (fastest? slowest?).
Problem RK5.3.
Comparing the strengths of bonds that will be broken in a reaction is often a good way to get a first estimate of relative activation barriers.
a) Use the following bond strengths to estimate the barriers to addition of a nucleophile (such as NaBH4) to the following double bonds: C=O (180 kcal/mol); C=N (147 kcal/mol); C=C (145 kcal/mol). Make a sketch of the three reaction progress diagrams.
b) In general, C=O bonds are the most reactive of these three groups toward electrophiles, followed by C=N bonds. Are these relative barriers consistent with this observation?
c) What other factor(s) might be important in determining the barrier of the reaction?
d) Modify your reaction progress diagram to illustrate these other factors.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic,
Biological and Inorganic Chemistry by
Chris Schaller
is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Web Materials on Structure & Reactivity in Chemistry