
Reactivity in Chemistry
Fatty Acid Synthesis
FA2. Transformations in Fatty Acid Synthesis
When we looked earlier at the citric acid cycle, we saw that acetyl coenzyme A, or AcCoA, was a key link between that process and glycolysis. This two-carbon remnant of glucose metabolism undergoes additional metabolic steps that harness energy in the form of ATP. But that's not the only purpose of AcCoA; it also serves as a building block for other compounds that are necessary for the survival of the cell or the organism.
Take myristic acid as an example, and think about how simple building blocks like AcCoA might be used to make it. AcCoA is a thioester, much like an ester but with a sulfur in place of one of the oxygen atoms. If we need to build myristic acid up from smaller pieces, we are going to need carbon-carbon bond-forming steps or homologation steps. You may not realise it, but there aren't an awful lot of those; their number just doesn't compare to the number of reactions that will simply switch out one heteroatom for another, such as an oxygen for a nitrogen. So, there may be a couple of choices, but not many.
The obvious carbon-carbon bond-forming reaction to perform with an ester is a Claisen condensation. In a Claisen condensation, the leaving group attached to the carbonyl is replaced by an enolate nucleophile. If the enolate nucleophile comes from AcCoA, then two more carbons will be added to the structure of the electrophile. So, if we were going to add two carbons to something in order to get myristic acid, it should be something with twelve carbons, like lauric acid.
Remember, the open arrow (below) means "comes from", so this picture means the target compound, myristic acid thioester, could come from the two synthons on the right.

Figure FA2.2. Myristic acid thioester and its precursors.
This isn't exactly how it happens in the cell, but there are a couple of complications in the actual biosynthesis and we will work our way up to them.
Retrosynthetically, we could think of myristic acid (or the corresponding thioester, easily hydrolysed to give the free fatty acid) as potentially being made from two simpler synthetic precursors: lauric acid (or its thioester) and the enolate ion of AcCoA. The AcCoA would add another two carbons to the chain of the lauric acid, making a fourteen-carbon chain.
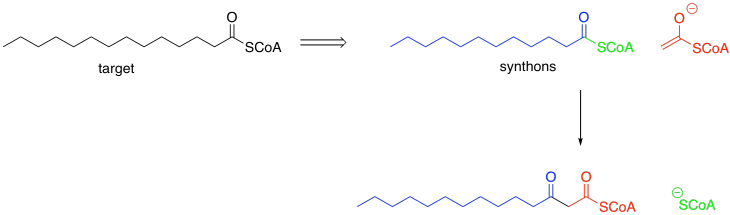
Figure FA2.3. Myristic acid's precursors first react to make a β-ketothioester.
Problem FA2.1.
Show the product of a Claisen condensation between the enolate anion of acetyl CoA and another molecule of acetyl CoA.
Where would the lauric acid come from? We will need to look further into that question, but you may already have thought of another problem that we need to solve, first: Although a Claisen condensation of lauric acid thioester and AcCoA enolate would result in a two-carbon chain extension, it would also bring an unwanted oxygen into the structure. There is a missing link between this structure and myristic acid. That oxygen needs to be replaced by a pair of hydrogens.

Figure FA2.4. A carbonyl oxygen of the β-ketothioester must be replaced by hydrogen atoms.
We can certainly get started by replacing one of the C=O bonds with a C-H bond. That should bring to mind a familiar carbonyl reaction. In the lab, we could use a hydride reagent, such as sodium borohydride (although we would have to worry about whether that thioester might react, too). In the cell, the equivalent reagent would be another hydride donor such as NADH.
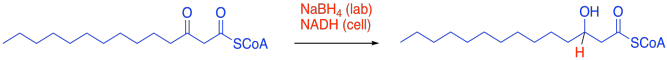
Figure FA2.5. Hydride reduction of the β-ketothioester gives one new hydrogen atom and leaves one C-O bond.
That gets us part of the way there. How do we get rid of the other bond to oxygen? How do we add a second hydrogen? The first question might be answered by thinking about something else that might be familar: an aldol addition. The reason that might be relevant is that the compound now contains a beta-hydroxy carbonyl. We see something similar in an aldol addition. Aldol additions frequently result in beta-hydroxy carbonyl units. However, they sometimes proceed though a subsequent elimination reaction, the loss of water to give an enone ("een-own"). That variation is sometimes called an aldol condensation to distinguish the two outcomes, although the two terms are used loosely.
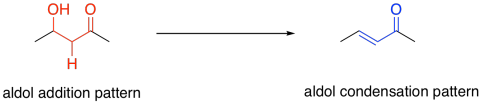
Figure FA2.6. The aldol condensation pattern.
Problem FA2.2.
What is the driving force for the dehydration reaction (loss of water) after the initial aldol addition?
In laboratory chemistry, that condensation often results in the presence of strong base and high heat -- conditions that might be a little hard on a cell. We'll find that the reaction conditions are much milder in the cell.
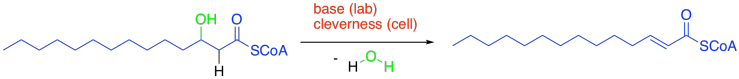
Figure FA2.7. Elimination to form the α,β-unsaturated thioester.
Problem FA2.3.
What thermodynamic factor leads increased heat to promote the dehydration reaction?
Now the problem of replacing that extra oxygen with two hydrogens is almost solved. The first hydrogen was added as a hydride, and that would work for the second one, too. This time, we need a 1,4-addition rather than a regular 1,2-addition. In the lab, that might be helped by adding a Lewis acid under the right conditions. In the cell, it might be aided by positioning of the hydride reducing agent in the right place in the enzyme, so that it delivers the hydride to the right place.
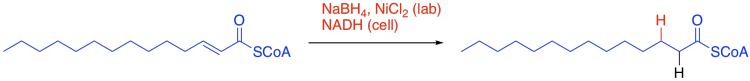
Figure FA2.8. 1,4-addition of hydride to form the saturated thioester.
If we put all of those steps together, we can get from lauric acid thioester to myristic acid thioester in just a few steps.
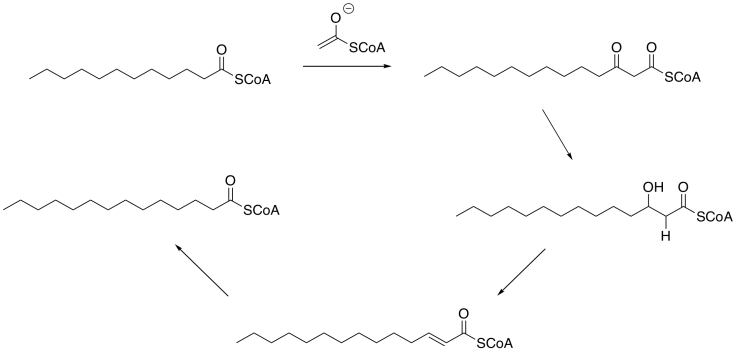
Figure FA2.9. Conversion of lauric acid thioester to myristic acid thioester.
Hold onto that thought, because we can use it again to make lauric acid from one that is two carbons shorter. In fact, we can keep repeating this cycle over and over until we work our way back to acetyl coenzyme A. Two molecules of AcCoA, one acting as the electrophile and the other as the enolate, could be used to make a four-carbon thioester. Adding another AcCoA would make a six-carbon thioester, and so on.
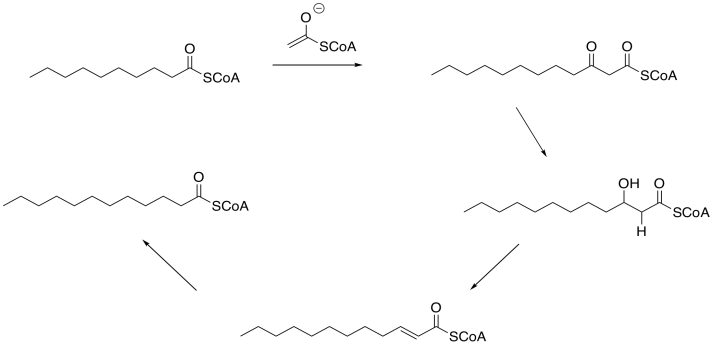
Figure FA2.10. Conversion of capric (decanoic) acid thioester to lauric acid thioester..
This dependence on a two-carbon building block is the reason for the vast preponderance of even-numbered carbon chains in natural fatty acids.
Next, we will take a look at how the cell actually shepherds these molecules through these kinds of reactions.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic,
Biological and Inorganic Chemistry by
Chris Schaller
is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: