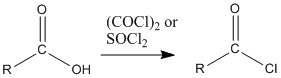
Figure CX5.1. Possible syntheses of an acid chloride.
Reactivity in Chemistry
Substitution at Carboxyloids
CX5. Getting Towed Uphill
The uphill carboxyloids are useful materials in terms of being able to make other compounds. For example, a thioester such as acetyl coenzyme A may be able to make a variety of acyl esters. In the laboratory, acid chlorides are very common starting materials to make other carboxyloids. However, if they are so far uphill, how are they formed in the first place?
The two most common methods of making acid chlorides are treatment of a carboxylic acid with thionyl chloride or with oxalyl chloride.
Figure CX5.1. Possible syntheses of an acid chloride.
The key part of making the uphill acid chloride out of the downhill carboxylic acid is the reagent used. Structurally, the reagents can be compared to acid chlorides themselves. They can be thought of as being a little bit like uphill carboxyloids themselves. Thus, as one compound gives its chloride and gets oxygenated on its way downhill, it provides the energy needed to drive the carboxylic acid uphill.
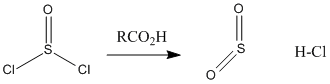
Figure CX5.2. Conversion of thionyl chloride to sulfur dioxide and hydrochloric acid.
Oxalyl chloride has some rough structural similarity to thionyl chloride. Both compounds are kind of like acid chlorides, which also makes them higher in energy like acid chlorides. In fact, you can think of them as being even more reactive than acid chlorides.
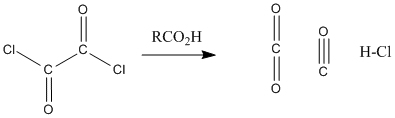
Figure CX5.3. Conversion of oxalyl chloride to carbon dioxide, carbon monoxide and hydrochloric acid.
Acid chlorides are highly electrophilic kinetically, partly because of the amount of positive charge on the carbonyl carbon. That factor is even more pronounced in oxalyl chloride and thionyl chlroide because of the additional nearby electronegative atoms. In addition, both of these compounds are thermodynamically unstable with respect to their products. It is easy to decompose thionyl chloride into sulfur dioxide and hydrogen chloride, or oxalic acid into carbon dioxide, carbon monoxide, and hydrogen chloride. Both of the compounds are very sensitive to moisture for this reason.
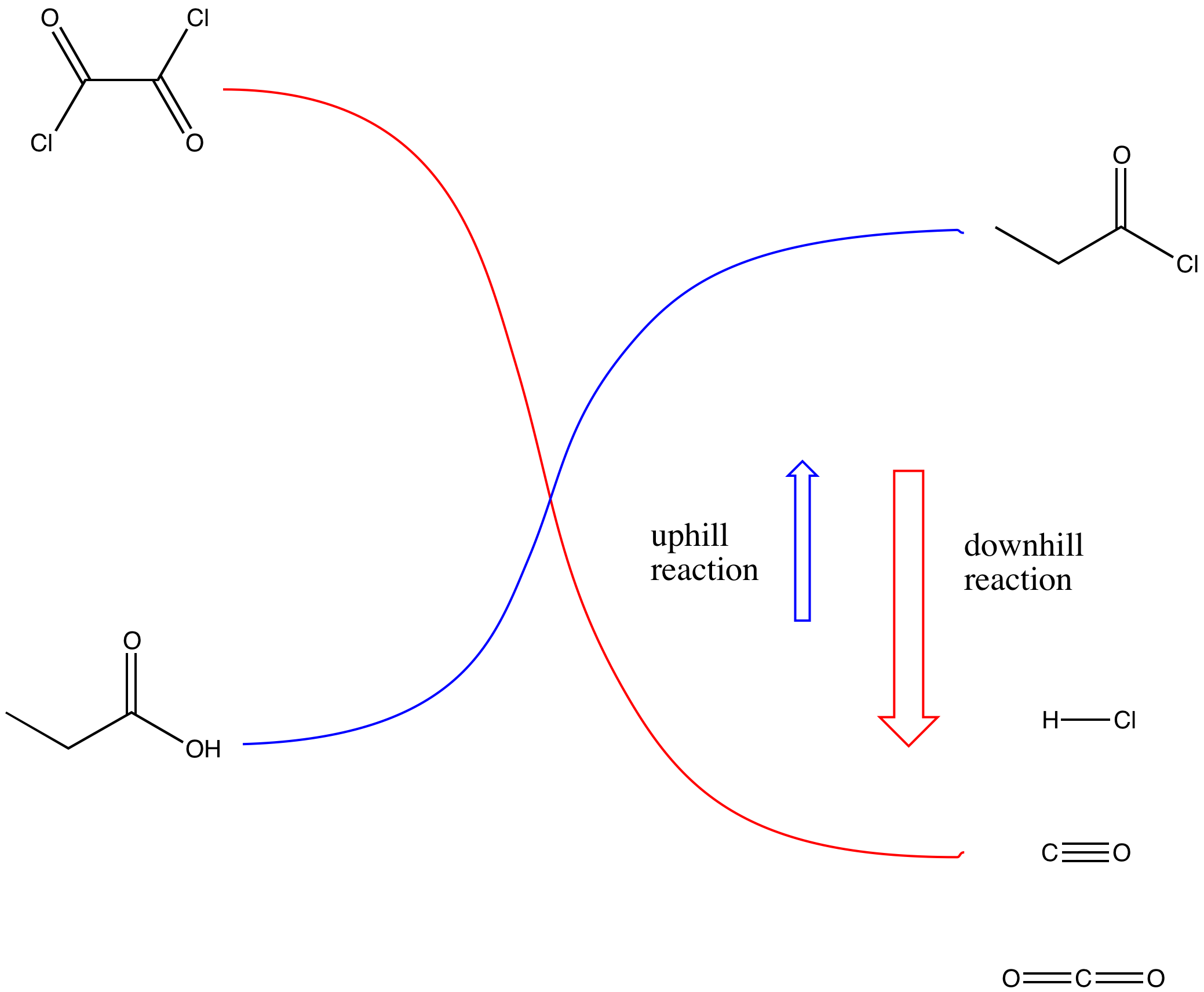
Figure CX5.4. The uphill reaction is driven by a coupled downhill reaction.
Problem CX5.1.
Reaction of a carboxylic acid with oxalyl chloride starts with a carboxyloid substitution using the oxalyl chloride as electrophile and carboxylic acid as nucleophile. The chloride ion that is liberated then acts as a nucleophile in a cascade reaction that releases CO2 and CO as an acid chloride forms. Draw the mechanism.
Problem CX5.2.
Reaction of a carboxylic acid with thionyl chloride is very similar to reaction with oxalyl chloride, described above. Draw the mechanism of the reaction.
Problem CX5.3.
Provide additional factors (such as energetics, equilibrium concepts) that explain why oxalyl chloride and thionyl chloride can drive the conversion of a carboxylic acid to an acid chloride.
Acid anhydrides are usually made from the corresponding carboxylic acids. One molecule of carboxylic acid acts as a nucleophile and a second acts as an electrophile. Because the same kind of molecule acts as nucleophile and electrophile, acid anhydrides are typically symmetric: they havae a (C=O)O(C=O) unit in the middle, with the same alkyl groups on either side of it.
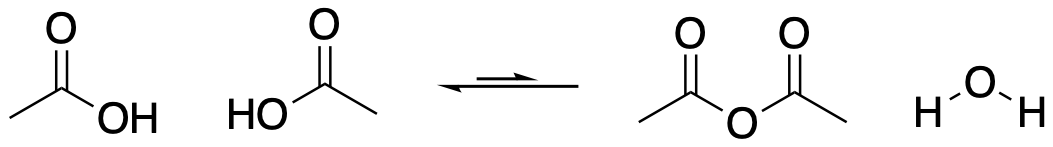
Figure CX5.5. Anhydrides form by loss of water from a carboxylic acid.
To aid formation of acid anhydrides, carboxylic acids are often heated strongly (well above the boiling point of 100 oC, usually several hundred degrees). Otherwise, they are sometimes heated more gently in the presence of a strong drying agent, such as phosphorus pentoxide (empirically, P2O5). In the presence of water, phosphorus pentoxide is converted to phosphoric acid, H3PO4.
Problem CX5.4.
Draw a mechanism for the conversion of ethanoic acid to ethanoic anhydride.
Problem CX5.5.
Show the reverse reaction to the conversion of ethanoic acid to ethanoic anhydride (the same reaction, in the other direction). Which of the two directions do you think is favourable, based on the ski hill?
Problem CX5.6.
Draw a mechanism for the conversion of phosphorus pentoxide to phosphoric acid.
Problem CX5.7.
Explain why the conditions outlined above lead to acid anhydride formation.
Problem CX5.8.
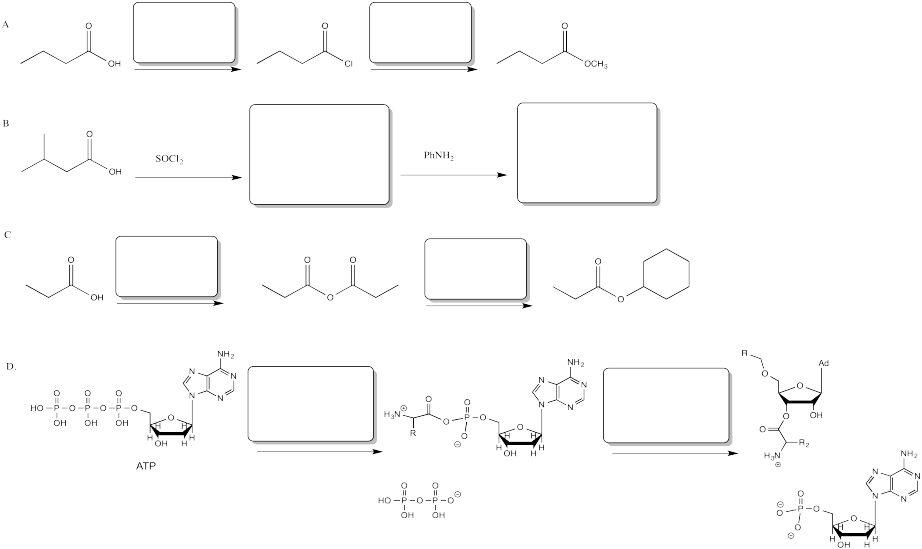
Fill in the blanks in the following problem.
Answers to selected problems are found here.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and
Inorganic Chemistry by
Chris Schaller is licensed under a
Creative
Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Carboxyl Substitution Index
Back to Web Materials on Structure & Reactivity in Chemistry