CO12a. Enolate Ions
Enolate ions are just another example of anionic carbon nucelophiles. The reason they get a page to themselves is that they are especially important, especially in biological chemistry. They are also important in the synthesis of organic compounds, such as in the pharmaceutical industry.
Forming Enolate Ions
An enolate ion is the anion that forms when a proton is removed next to a carbonyl. The carbon next to the carbonyl is called the α-position (alpha position). The alpha position is acidic both because of the amount of positive charge on a proton in that position and because of the stability of the anion that results if that proton is removed.
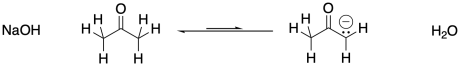
Figure CO12a.1. Formation of an enolate ion at equilibrium.
- A hydrogen on a carbon next to the C=O bond is called an α-hydrogen.
- These protons can be removed by strong bases.
- The anion left behind is called an enolate anion.
You might have learned about metal hydroxides such as sodium hydroxide and lithium hydroxide. The metal-oxygen bond is ionic because of the large electronegativity difference between the metal and the oxygen. These compounds give rise to hydroxide ions. Those hydroxide ions are basic because they can easily pick up protons to become neutral water molecules. Metal hydroxides are commonly seen in chemistry, and they are thought of as strong bases.
Other oxygen anions are also able to act as strong bases, unless there is some resonance factor that delocalises the anion and makes it less reactive. Methoxide ion and butoxide ion are also common strong bases.
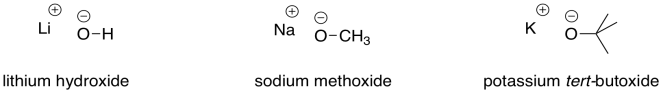
Figure CO12a.2. Some strong bases.
Problem CO12a.1.
Show why an enolate ion, such as the one formed from 2-propanone, above, is particularly stable.
Problem CO12a.2.
Show a mechanism, with curved arrows, for the formation of the enolate ion from 2-propanone, above.
Other ketones and aldehydes can be deprotonated just like 2-propanone. The proton is always removed from the α-position, from the carbon next to the carbonyl. Even in an aldehyde, such as pentanal, the proton is removed from the α-position; the aldehyde proton, RC(O)-H, is not as easily removed by a base as that proton next to the carbonyl.
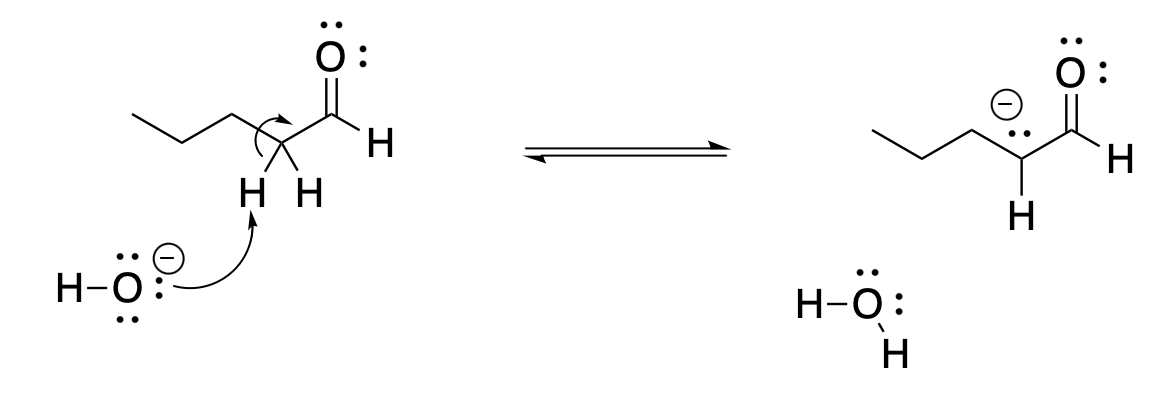
Figure CO12a.3. Mechanism showing proton removal from the α- position of pentanal.
The peculiar acidity of that specific C-H bond can be explained by the stability of the anion that is formed when the proton is lost. A lone pair and anion in the α-position can be delocalized by resonance. Remember, charge delocalization is generally stabilizing. Enolate ions are more stable than most carbon anions, especially because the negative charge is shared with a more electronegative oxygen in this case. Notice that both resonance structures have octets on all of the atoms; they are both good resonance structures.
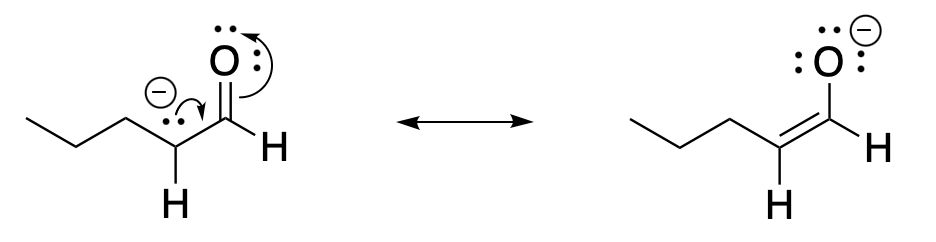
Figure CO12a.4. Resonance delocalization of an enolate anion.
That means there is actually a second way we can show the mechanism for the reaction, taking into account the second resonance structure. This second way is useful to keep in mind, because it gives us information that we don't see in the first drawing of the mechanism. This way, we can see the contribution that the oxygen makes in stabilizing the anion.
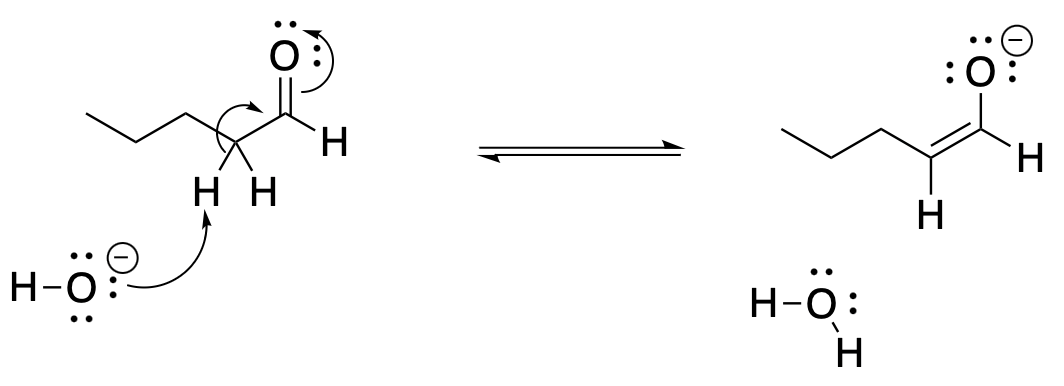
Figure CO12a.5. Another way to show the mechanism for the formation of an enolate anion.
In the examples above, the ketone is deprotonated at the α position to form the corresponding enolate ion. However, sodium hydroxide is not a strong enough base to convert all of the ketone to its enolate. The resulting enolate is basic enough to pull a proton from a water molecule, so an equilibrium results.
That would be the case any time a strong base such as a hydroxide or an alkoxide was used to deprotonate a ketone or aldehyde. In the following example with pentanal, the reaction would also result in an equilibrium between the reactants and the products.

Figure CO12a.6. Equilibrium between aldehyde and enolate using strong base.
That means that all of those compounds on both the left hand and right hand side of the arrow would all be present as a mixture.
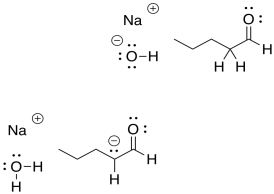
Figure CO12a.7. An equilibrium mixture contains all species involved in the reaction.
- Even strong bases like hydroxide can only remove a fraction of the α-protons in a kaetone.
- The enolate ion exists in equilibrium with the ketone.
Negative charges are fairly stable on oxygen atoms. That allows this particular reaction to shift back to the left again, to form that hydroxide ion again. To make the reaction go all the way to the right, we would need a less stable anion on the left. That would make that anion more basic. Can you think of atoms that would be less stable as anions than oxygen?
The most commonly used very strong bases in synthetic chemistry involve anions of carbon, nitrogen or hydrogen. Some examples of compounds used as very strong bases are sodium hydride (NaH), sodium amide (NaNH2), lithium diisopropylamide (LiN[CH(CH3)2]2, also called LDA for short), and butyllithium (CH3CH2CH2CH2Li, abbreviated BuLi).
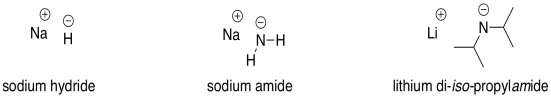
Figure CO12a.8. Some very> strong bases.
In all of these compounds, the negative charge is on a less electronegative element than the oxygen of the hydroxide ion. That means that they are less stable and more reaction than hydroxide.
If one of these bases were to react with an aldehyde or ketone, the proton would be removed irreversibly. The delocalised enolate ion is actually more stable than the original amide ion in sodium amide, for example.

Figure CO12a.9. Formation of an enolate ion at equilibrium.
As a consequence, adding a very strong base to an aldehyde or ketone results in complete conversion into products. At the end of the reaction, there are no reactants left.
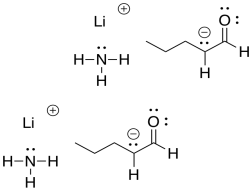
Figure CO12a.10. A very strong base leads to all products, no reactants.
In contrast, a "strong base" such as sodium hydroxide won't really do the job. If it did, we would be trading in an anion on a more electronegative atom (oxygen) for an anion on a less electronegative atom (carbon) in the same row of the periodic table. That's not possible. The enolate anion that forms would be more basic than the hydroxide we began with, and most of the time it would just snatch the proton back from the water again, making ketone and hydroxide again.
-
Enolate ions form in equilibrium with their parent carbonyl compounds if a moderately strong base like sodium hydroxide is used.
-
A very strong base, like sodium amide (NaNH2), butyllithium (CH3CH2CH2CH2Li, or BuLi) or sodium hydride (NaH), would result in complete enolate formation.
However, it is sometimes really useful to have an equilibrium between a carbonyl compound and its enolate. That situation allows both a ketone (the 2-propanone, left) and its enolate (right) to be present at the same time. That means there is both a nucleophile and an electrophile (the ketone and the enolate). They will be able to react together.

Figure CO12a.11. Formation of an enolate ion at equilibrium.
While we are on the subject of bases, there is a third category of compounds that we would consider weak bases. The most common examples are amines (but not amides) and resonance-stabilised oxygen anions.
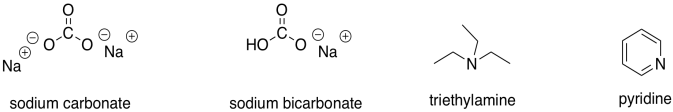
Figure CO12a.12. Some weak bases.
These compounds would be good at picking up excess protons that were floating around. However, in most cases they wouldn't be strong enough bases to provide an appreciable amount of enolate ion.
Problem CO12a.3.
Identify the following compounds as weak, strong, or very strong bases.
In the next section, will look at enolate nucleophiles reacting with carbonyl electrophiles. These reactions are called aldol reactions. Aldol reactions are important because they provide another way of making carbon-carbon bonds. That proves to be pretty useful in building up larger carbon structures. In nature, these reactions are used to build carbohydrates from smaller molecules.
Solutions to selected problems
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and
Inorganic Chemistry by
Chris Schaller is licensed under a
Creative
Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Carbonyl Addition Index
Back to Web Materials on Structure & Reactivity in Chemistry