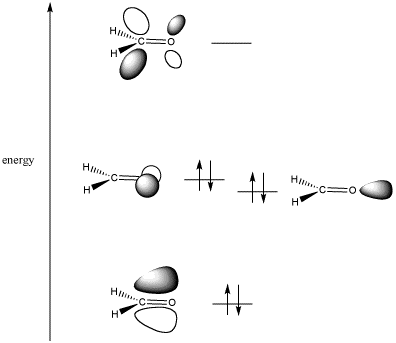CO5. Molecular Orbital Picture of Carbonyls
Often it is useful to look at the molecular orbital picture of a molecule to learn something about its reactivity.
In the case of carbonyls, frontier orbital ideas tell us to look at the lowest unoccupied molecular orbital (LUMO) and the highest occupied molecular orbital (HOMO).
Figure CO5.1. Frontier orbitals in a carbonyl compound.
When two different atoms bond together, the molecular orbitals that they form are not evenly distributed between the atoms. Instead, the new molecular orbital is closest in space to the atom to which it is closest in energy.
In the case of carbon and oxygen, oxygen is more electronegative than carbon. That means its electrons are more tightly held than carbon's. That means its electrons are lower in energy than carbon's.
When carbon and oxygen combine, a bonding orbital and an antibonding orbital result. The bonding orbital is lower in energy than the orbitals on either carbon or oxygen. However, it is closer in energy to oxygen. Thus, the orbital itself is more centered on oxygen. In other words, the electrons in the bond are closer to oxygen than to carbon.
The antibonding orbital, on the other hand, is closer to carbon in energy, although it is higher in energy than either carbon or oxygen. It is more centered on the carbon than the oxygen. That means the "target" for the electron donation is mostly found on the carbon. The carbonyl carbon is the electrophilic position.
If electrons are going to be donated to the molecule, the lowest energy position available for electrons in the molecule is described by the LUMO. The LUMO in this case is the C=O pi* or pi antibonding orbital.
- A nucleophile will donate a pair of electrons into the C-O π* orbital of the carbonyl.
- This orbital is mostly based on carbon.
If the carbonyl is going to donate electrons, the
electrons will come from the HOMO. In this case, that refers to the non-bonding
electrons. These electrons are found on the oxygen, and are equivalent to the
lone pairs in the Lewis structure.
Problem CO5.1.
Explain what happens to bonding in the molecule if an electron pair is donated to the LUMO of H2C=O.
Problem CO5.2.
Draw the frontier-orbital diagram (including orbital pictures) for the following compounds.
a) CH3OH b) H2C=NH c) CH3CN
Problem CO5.3.
In the previous problem, which MO pictures most closely resemble the one shown for H2C=O? Which compounds will behave most like a carbonyl compound? Explain.