
Structure & Reactivity
Nuclear Magnetic Resonance Spectroscopy
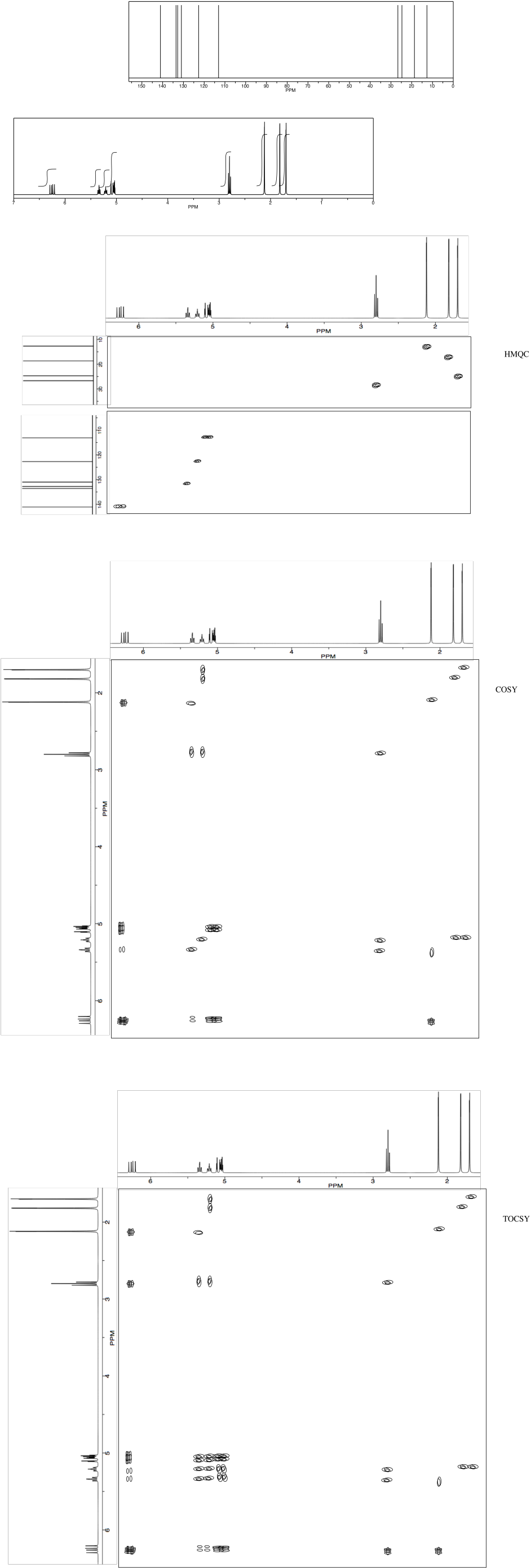
NMR2D.2. TOCSY
Total Correlation Spectroscopy, or TOCSY, is almost exactly the same as regular COSY. However, instead of just showing that two protons are coupled, it shows an entire spin system together. That means it shows this proton is connected to that proton, but that proton is connected to this other one, which is connected to that one over there. A TOCSY spectrum displays an entire chain of protons, each of which is coupled to the next.
As an example, we'll look at ethoxypropane. It has a two-carbon chain and a three-carbon chain, but the two chains are separated from each other by an oxygen atom.

The 1H NMR spectrum is shown below. It isn't all that complicated. However, if we wanted to know exactly which peak belonged to which hydrogen, we may have some trouble. In particular, how could we conclusively tell those two triplets apart near 1 ppm? An educated guess would put the methyl of the ethyl chain downfield, farther to the left. Can we confirm that suspicion?
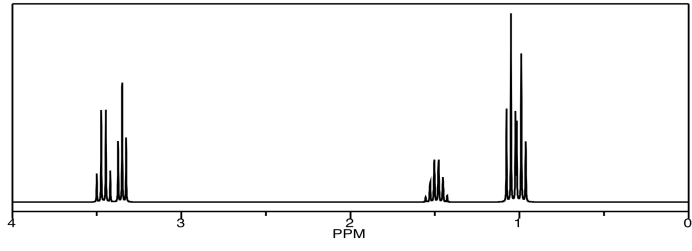
The TOCSY spectrum shows what we need to see in order to be sure. These spectra don't really show up colour-coded, but red and blue have been added to make things more clear. The blue part is exactly what we would see in a COSY spectrum. The hydrogen at 1.1 is coupled to the one at 3.4. The red part is slightly different from the COSY; not only does it show the coupling between the hydrogen at 1.0 and the one at 1..5, but it also includes the hydrogen at 3.3, because that one is also coupled to the one at 1.5. The entire chain of coupled hydrogens is linked together.
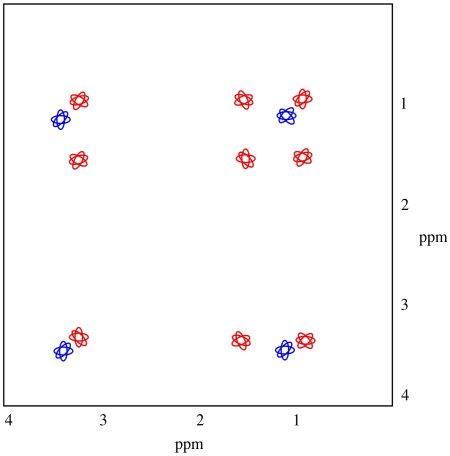
At a glance, the TOCSY spectrum tells us we have a two-carbon chain because we see two peaks in a row (or, really, a two by two array). It also tells us we have a chain of three peaks because we can see three peaks in a row (or a three by three array).
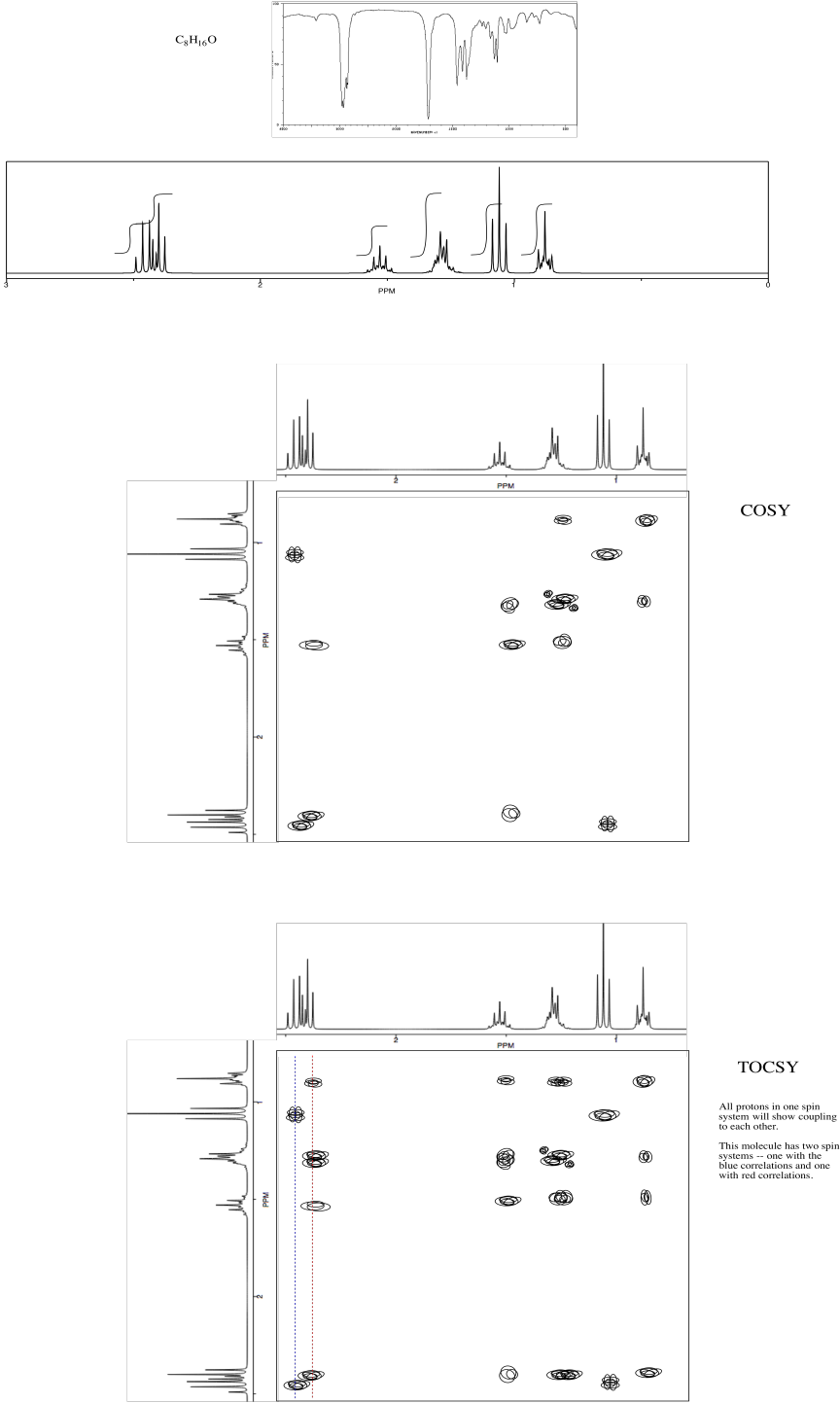
Problem NMR2D.2.1.*
Identify the compound.
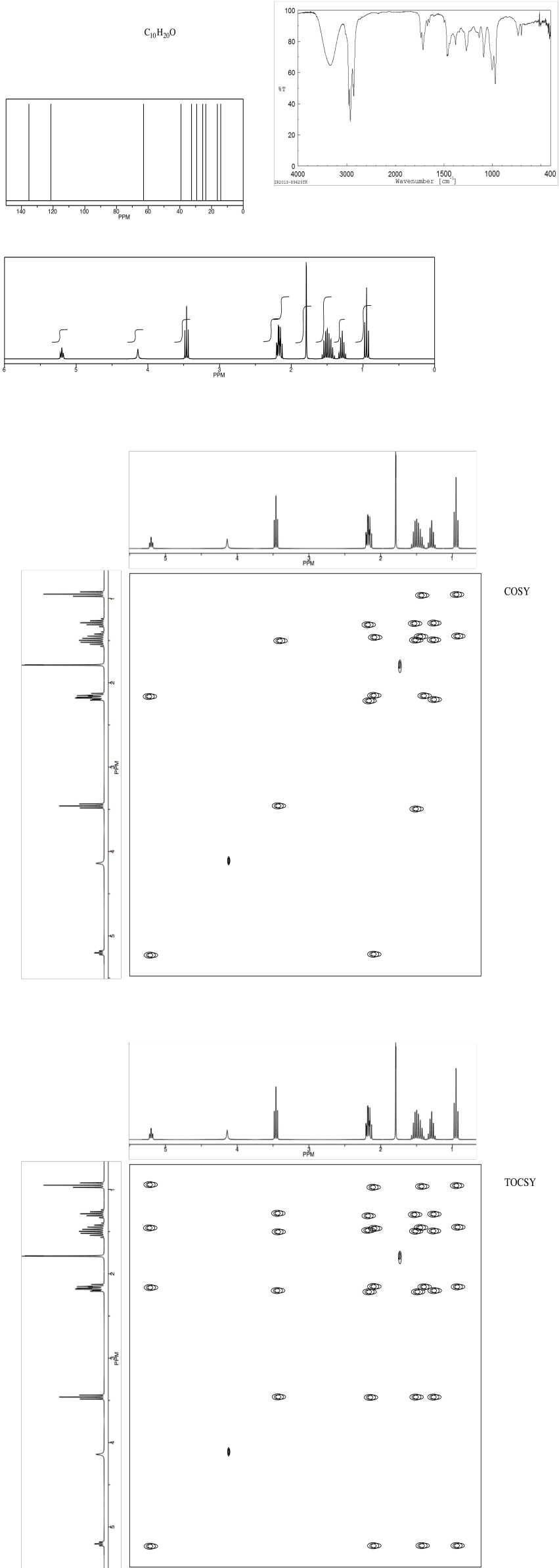
Problem NMR2D.2.2.*
Identify the compound.
Problem NMR2D.2.3.*
Identify this compound.
*Sources:
Selected IR spectra from SDBS (National Institute of Advanced Industrial Science and Technology, Japan, Spectral Database for Organic Compounds, http://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_index.cgi, accessed December, 2015).
1H NMR, 13C NMR, TOCSY and COSY spectra simulated.
Contributions from Kate Graham, College of Saint Benedict | Saint John's University
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation:
Useful Charts for NMR identification.