SC11. Carbohydrates in cyclic form
Carbohydrates are complicated molecules. In solution, they slowly change into different isomers. In water, most carbohydrates can change from one form to another quickly; they are described as being in equilibrium with different structures. That means they can change back and forth.
The most prevalent form for most carbohydrates is a ring. One of the oxygens farther along the chain can reach around and bond to the carbon in the C=O at the head of the chain. When that happens, there are two possible orientations of the oxygen at the head of the chain. The two stereoisomers that result are diastereomers. Some of the chiral centers are the same, but one is different.
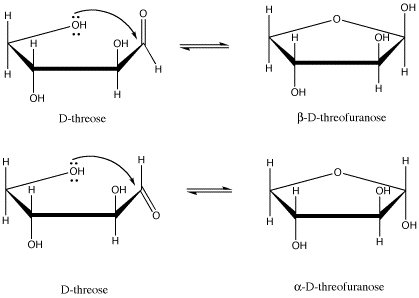
Figure SC11.1. D-threose and its two possible five-membered-ring forms.
For example, D-threose can form two different five-membered rings (that is, rings made from a circle of five atoms). In carbohydrates, a five-membered ring with an oxygen in it is called a furanose (another common form, a six-membered ring, is called a pyranose). There are two diastereomers formed. they are sometimes called anomers. That means they differ in 3D space at the anomeric center; the anomeric center is the C=O carbon to which an oxygen binds to form the ring.
These drawings, by the way, are called Haworth projections. They are commonly used in biochemistry to depict the cyclic forms of sugars. They seem to suggest a funny see-saw shape for the carbon atoms, but the idea here is not to convey the shape of the molecule perfectly. Just like in Fischer projections, the main concern is to quickly convey stereochemical relationships. In a Haworth projection, you can easily see whether two groups are cis to each other or trans to each other.
Note that a Haworth projection of an odd-numbered ring is always drawn with the ring oxygen in the back. We are looking at the ring from the edge and slightly above, so the "back" is the upper edge of the polygon. It's just like if you were looking at a swimming pool, and the far corner of the pool appeared in your field of vision above the near corner. For an even-numbered ring, the same idea applies, but the ring oxygen is always drawn on the top edge and to the right.
If you want to get an idea of the true shape of the molecule, you can see it in the ball-and-stick models below.
Figure SC11.2. Ball-and-stick model of β-D-threofuranose.
Figure SC11.3. Ball-and-stick model of α-D-threofuranose.
Go to Animation SC11.1. A three-dimensional model of β-D-threofuranose.
Go to Animation SC11.2. Another three-dimensional model of α-D-threofuranose.
- Carbohydrates are normally found in their cyclic forms, not their chain forms.
- For every ring a carbohydrate can form, there are always two diastereomeric forms called anomers.
The two terms, α and β, that described the two different stereoisomers of the cyclic form of threose refer to the position of the OH group on the newly formed chiral center (the old C=O was trigonal planar, not tetrahedral, so it wasn't chiral). The terms are defined by looking at the Haworth projection of the molecule. If the new OH is in the upper position, it is termed β (think butterfly); it is is in the lower position, it is termed α (think ant).
Carbohydrates do not always make five-membered rings. Sometimes they make six-membered rings instead. A five-membered ring with an oxygen in it is called a furanose. A six-membered ring with an oxygen in it is called a pyranose. Ribose is a five-carbon sugar that could make either a five-membered or a six-membered ring, although we usually see it in its five-membered ring.
Figure SC11.4. Ribose in equilibrium between its chain form and some of its ring forms.
The presence of different diastereomers that can change back and forth complicates things. If you wanted to measure the optical activity of a sugar, you would probably open a bottle of the solid carbohydrate, dissolve some up in water and put the sample in a polarimeter. However, the optical rotation would change over time as the pure solid slowly turned into other isomers. This process is called mutarotation (which means changing optical rotation). For this reason, the time at which a measurement was performed is often reported with optical rotation values of carbohydrates. For example, the optical rotation value for D-threose may be listed as [α]D -12.3 (20 min, c = 4, water).
Carbohydrates are important partly because they are structural units in biology. Carbohydrates are incorporated into DNA and RNA as well as important enzymatic cofactors such as ATP, NADH and Acetyl Coenzyme A. Very frequently, the unique pieces attached to the carbohydrates to make these different molecules are attached via the anomeric carbon.
- Specific OH groups on carbohydrates are frequently substituted with other groups to make more complex molecules.
One more piece of terminology may be useful to review here. There are terms used in ring structures to describe whether two groups are attached to the same face or on opposite faces. For example, in β-D-threofuranose, the second hydroxy group is cis to the one on the anomeric center, but in α-D-threofuranose, the second hydroxy group is trans to the one on the anomeric center.
- Two groups on the same face of a ring are described as being cis to each other.
- Two groups on the opposite faces of a ring are described as being trans to each other.
- The terms cis and trans can be applied to any rings, not just carbohydrates.
Problem SC11.1.
In the following carbohydrate, glucose, show the cyclic structures that would form if the indicated oxygen bonded to the carbonyl carbon (the C=O in the CHO group at the top). You should move a proton from one oxygen to another so that all the atoms have the usual number of bonds.
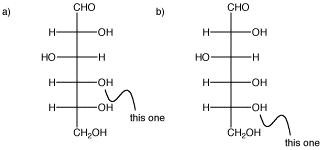
Problem SC11.2.
β-deoxyribofuranose (below) is a building block of DNA.
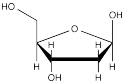
a) What is the relationship between the two hydroxy (OH) groups that are directly attached to the 5-membered ring?
b) In the other possible 5-membered ring form of deoxyribose, what is the relationship between the two hydroxy (OH) groups that are directly attached to the 5-membered ring?
