PM8. Liquid Chromatography
Solvent partitioning involves an equilibrium between dissolving in one liquid and dissolving in another. Our first look at chromatography involves a similar equilibrium between dissolving in a liquid and sticking to a solid.
Sticking to a solid, or adhesion, occurs through intermolecular attractions between the solid and the compound adhering to it.
A grass stain on the knee of your jeans goes into the washing machine and enters into this sort of equilibrium. The equilibrium constant involving these materials (grass stain compounds such as chlorophyll, the cellulose in the cotton of the jeans, and the soapy water) determines whether your jeans will become clean or remain stained.
The great advantage of chromatography is flexibility. It does not matter whether the compound you wish to purify is a solid or liquid. Even gases can be purified by chromatography, as you will see in a later section. As long as the compound is able to dissolve in one liquid and stick to one solid, chromatography can be used to purify it.
There is a very similar type of chromatography called paper chromatography. In a typical paper chromatography demonstration, a sample of ink is spotted onto a piece of filter paper. The filter paper is made of cellulose, like jeans. The paper is dipped into a beaker of water. The water begins to wick up along the paper. As it water seeps up past the spot of ink, the spot begins to move. If the ink is made from a mixture of pigments, it separates out into different, coloured compounds.
Problem PM8.1.
Look at the structures of paper (cellulose) and water. Why is water able to spread up through a piece of paper that is dipped into it?
Problem PM8.2.
Why does the ink separate into different components as the water seeps up the paper?
In liquid chromatography, there is a solid that stays put, called the stationary phase, and a liquid that moves over the solid, called the mobile phase or the eluent.
The solid is usually silica (SiO2) or alumina (Al2O3). Both are polar compounds capable of hydrogen bonding. Usually they have hydroxyl groups on their surfaces.
The eluent is usually an organic solvent or mixture of solvents. The eluent can be more polar or less polar. It should not be so polar that it would dissolve the alumina or silica. If it did, the stationary phase would not stay put, but would move with the liquid phase. For that reason, methanol and water are not normally used as the eluent.
If the solid phase is stationary, then when compounds are absorbed onto the solid, they will not move either. If the liquid phase is moving, then when compounds dissolve in the liquid, they will move along, too. If there is an equilibrium between solid-phase adhesion and liquid-phase solution, compounds will spend some time moving and some time staying still.
Different compounds may have different equilibria between solution and adhesion. That means different compounds will spend different amounts of time moving or staying still. As a result, the compounds will separate from each other over time.
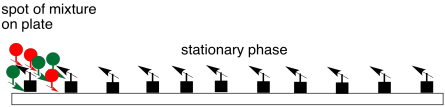
Schematically, chromatography works something like this. A mixture is placed at one end of the solid, stationary phase. In most cases, the stationary phase is polar; polarity is represented by arrows in the diagram. Think of the arrows as magnets. Chromatography is a little bit like a race; right now, everyone is at the starting line.

Figure PM8.1. Loading a mixture onto the stationary phase.
A liquid, mobile phase is made to flow across the stationary phase. Although the compounds in the mixture interact with the stationary phase and spend some time sticking to it, they will also interact with the mobile phase and spend some time dissolved in it. While they are dissolved in the mobile phase, they will move along with it, because it is flowing. The race begins.
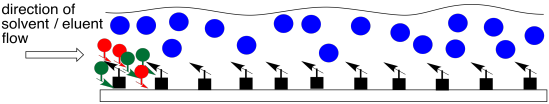
Figure PM8.2. Introduction of a mobile phase.
The two compounds in the mixture have different properties. They have different equilibrium constants governing how much time they spend in the liquid, mobile phase and how much time they spend on the solid, stationary phase. Eventually, the less polar compound pulls ahead of the more polar one. The more polar compound is spending more time sitting on the stationary phase. The less polar compound wins the race.

Figure PM8.3. Elution of the mixture along the plate.
Thin layer chromatography is often done as an exploratory method to quickly see whether a mixture of compounds can be separated based on their equilibria between a stationary, solid phase and a mobile, liquid phase. Thin layer chromatography is like paper chromatography, which is sometimes demonstrated in high school. A solid sheet or plate is dipped into a solution. As the solution moves up the surface of the solid, the compounds on the plate move along to a different extent based on their polarity.
A thin layer chromatography (TLC) plate can be made of metal, glass or plastic. The alumina or silica is sprayed onto the plate and it is allowed to dry, like paint. Very often, TLC plates are purchased already prepared, with the stationary phase already "painted" onto them. Sometimes students need to make the plates themselves.
The steps in a TLC experiment are outlined below.
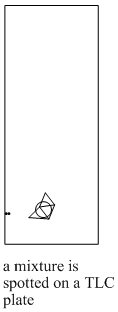
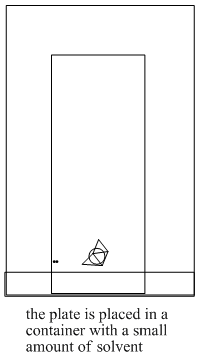
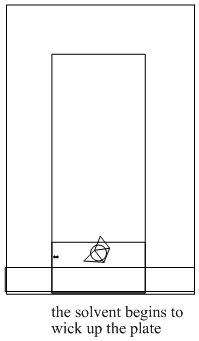
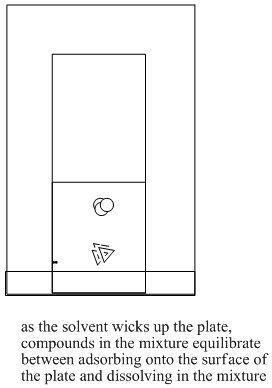
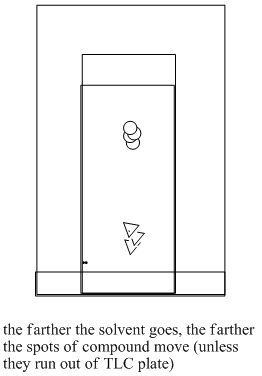
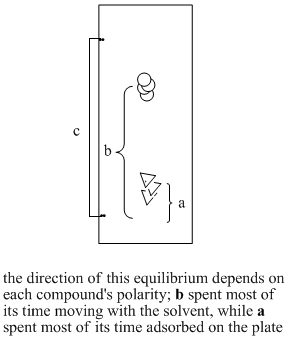
Figure PM8.4. Steps in running a TLC experiment.
Problem PM8.3.
Suppose you place a spot of sample on a TLC plate. You have pentane and 2-butanone to use as eluent. First you try the pentane. After the pentane elutes (or wicks up) all the way to the top of the plate, none of the compounds in your mixture have moved. What is wrong? How will you fix the problem?
Problem PM8.4.
Suppose you place a spot of sample on a TLC plate. You have pentane and 2-butanone to use as eluent. First you try the 2-butanone. After the 2-butanone elutes (or wicks up) all the way to the top of the plate, all of the compounds in your mixture have moved to the top of the plate, too. They have not separated from each other. What is wrong? How will you fix the problem?
Problem PM8.5.
Suppose you finally succeed in getting your mixture separated into three spots on a TLC plate. You want to isolate these three pure compounds and put them each in a labeled vial. Come up with a series of steps that you could do to accomplish this task.
TLC can also be used to help confirm the identity of a compound. If we suspect a mixture contains a certain compound, and you have a sample of that known compound on hand, we can load the TLC plate with two spots, side by side. When we elute the plate, we check to see whether one of the spots from the mixture has moved the same distance as the known compound.
Of course, this method is not foolproof. It could be that one of the compounds in the mixture just happens to move the same distance as the known compound, but is actually something else. Nevertheless, it is a relatively easy way to see whether you may be right.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Structure & Reactivity