Structure & Reactivity in Chemistry
Introduction to Molecules
IM8. Other kinds of drawings
Line drawings
Lewis structures can tell us a lot about how atoms come together to make molecules. They can also be cumbersome, especially if we are dealing with very large molecules. Drawing a line instead of a pair of dots for bonding pairs of electrons makes it easier to draw structures. There are other abbreviations that are helpful in some situations.
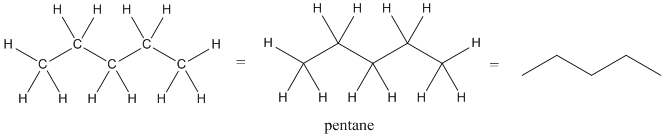
Because organic chemistry is based on the compounds of carbon, we would have to draw the letter C over and over again unless we had a shortcut. In line structures, we drop the label "C" for the carbon atoms. Anytime there is a joint between two bonds (drawn as a vertex in a zig-zag line), the atom attached to that bond is assumed to be a carbon unless written otherwise.

Figure IM8.1. Three ways of drawing pentane.
The atom at the end of a zig-zag line would also be a carbon, unless it is explicitly written as another atom.
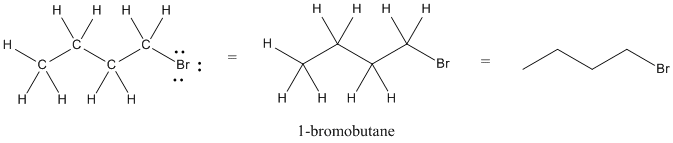
Figure IM8.2. Three ways of drawing bromobutane.
Taken even further, we will omit the hydrogens from our structures, since the compounds of carbon almost always contain hydrogen as well. Since we know carbon has a valence of four, we always know how many hydrogen atoms are attached to each carbon in order to reach that valence. A carbon with two bonds drawn in must have two hydrogens on it. A carbon with only one bond drawn to it must have three hydrogens.
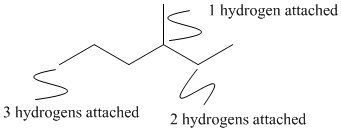
Figure IM8.3. The unlabeled hydrogens.
Note that the hydrogens are not normally omitted if they are attached to heteroatoms (atoms other than carbon, such as oxygen or nitrogen). It's important to show hydrogen in these cases because the number of hydrogen atoms can vary with these atoms. For example, a proton (H+) can sometimes be removed from an O-H bond. We need to know for sure whether that hydrogen is there or not. It is much less likely that we are missing a hydrogen on carbon, so we just assume those hydrogens are there.
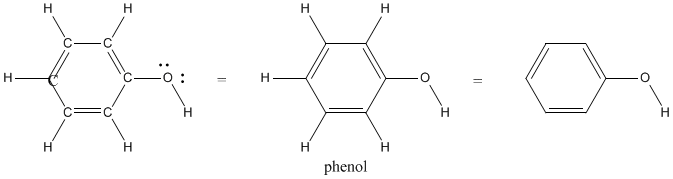
Figure IM8.4. Hydrogens on heteroatoms (O, N, S, P...).
You will also note that lone pairs are frequently left out when we use line structures, so you will have to add them back in to think about Lewis structures. Adding the lone pairs back to the heteroatoms in line structures is a good habit to get into, because later in the course we will be very concerned with keeping track of where all the electrons are.
Below is a summary, showing the relationship between Lewis/Kekule structures, line structures and condensed formulae for a few different compounds.
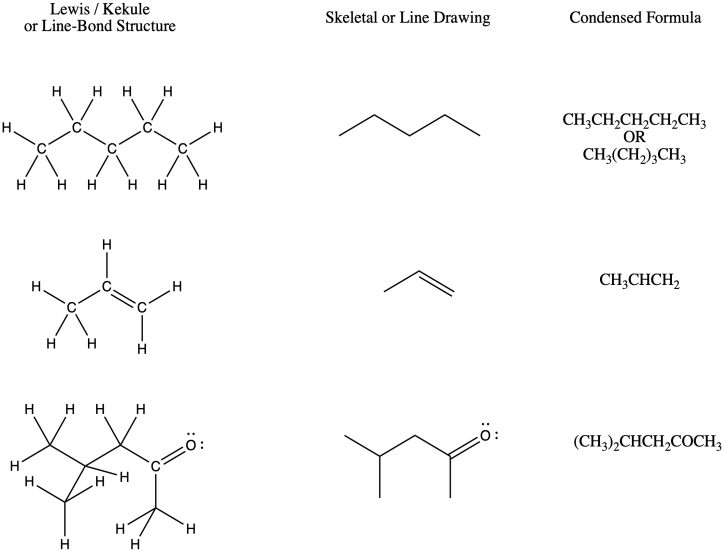
Figure IM8.5. Comparing different presentations of structures.
Remember, in phenol, the hydrogen attached to oxygen was labeled in the line structure. Sometimes there are exceptions in line structures, in which atoms that you might not think about labeling usually do get labels. The most common exceptions are shown below.
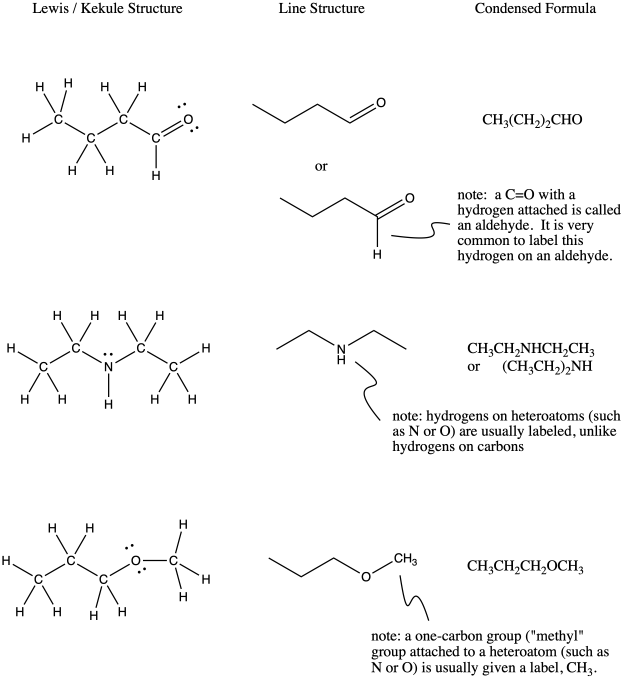
Figure IM8.6. Sometimes hydrogens should be shown.
Problem IM8.1.
Translate the following condensed formulae into line drawings.
a) CH3CH2NHCH2CH3 b) CH3CHFCH2CH2CH2Cl
c) CH2CHOCH2CH3 d) CH3CHClCH2SCH2CH3
Problem IM8.2.
Translate the following structures into condensed formula.
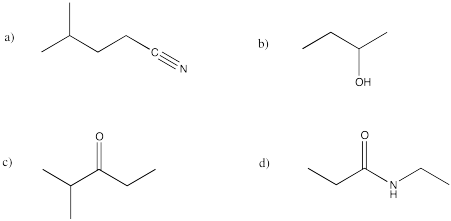
Problem IM8.3.
Try filling in the missing line drawing, Lewis / Kekule structures or condensed formulae in each line of the table below.
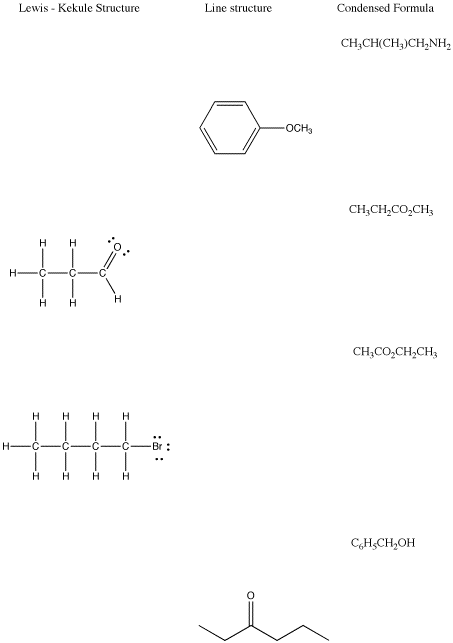
Problem IM8.4.
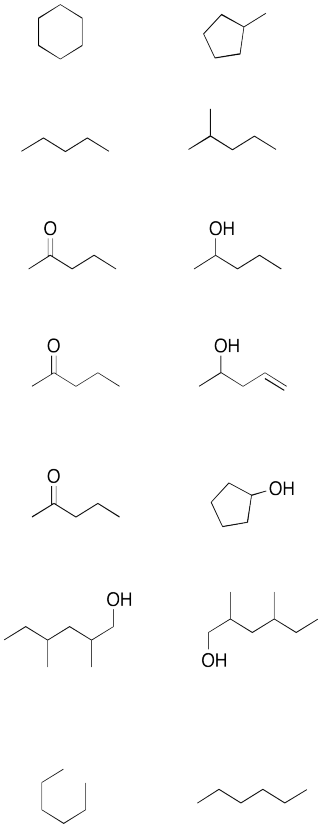
Look at the following pairs of compounds. Are the compounds constitutional isomers? (Hint: You may need to add in all hydrogens)
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure &
Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation:
Back to Structure & Reactivity Web Materials