Structure & Reactivity in Chemistry
Introduction to Molecules
IM7. Which bonds are ionic and which are covalent?
Cells contain lots of water. One of the roles of the water is to dissolve different materials. For example, there are many different ionic compounds (salts) in cells. Ions are used to maintain cell potentials and are important in cell signaling and muscle contraction.
How can you tell if a compound is ionic or covalent?
There is not a simple answer to this question. Many bonds are somewhere in between. In a polar covalent bond, a pair of electrons is shared between two atoms in order to fulfill their octets, but the electrons lie closer to one end of the bond than the other. There is more negative charge toward one end of the bond, and that leaves more positive charge at the other end.
Looking at the electronegativity values of different atoms helps us to decide how evenly a pair of electrons in a bond is shared. Electronegativity increases toward the upper right hand corner of the periodic table because of a combination of nuclear charge and shielding factors. Atoms in the upper right hand corner of the periodic table have a greater pull on their shared bonding electrons, while those in the lower left hand corner have a weaker attraction for the electrons in covalent bonds.
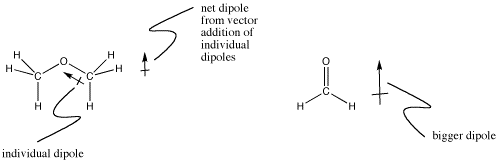
In a carbon-oxygen bond, more electrons would be attracted to the oxygen because it is to the right of carbon in its row in the periodic table. Compounds like , dimethyl ether, CH3OCH3, are a little bit polar. Formaldehyde, CH2O, is even more polar. Electrons in pi bonds are held more loosely than electrons in sigma bonds, for reasons involving quantum mechanics. That allows the oxygen to pull the electrons toward it more easily in a multiple bond than in a sigma bond.

Figure IM7.1. Dipoles in small molecules.
Not all polarities are easy to determine by glancing at the periodic table. The direction of the dipole in a boron-hydrogen bond would be difficult to predict without looking up the electronegativity values, since boron is further to the right but hydrogen is higher up. As it turns out, the hydrogen is slightly negative.
A bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. That situation is common in compounds that combine elements from the left-hand edge of the periodic table (sodium, potassium, calcium, etc.) with elements in the extreme upper right hand corner of the periodic table (most commonly oxygen, fluorine, chlorine). Sodium chloride is an ionic compound.
Many bonds can be covalent in one situation and ionic in another. For instance, hydrogen chloride, HCl, is a gas in which the hydrogen and chlorine are covalently bound, but if HCl is bubbled into water, it ionizes completely to give the H+ and Cl- of a hydrochloric acid solution. Even in gaseous HCl, the charge is not distributed evenly. The chlorine is partially negative and the hydrogen is partially positive.
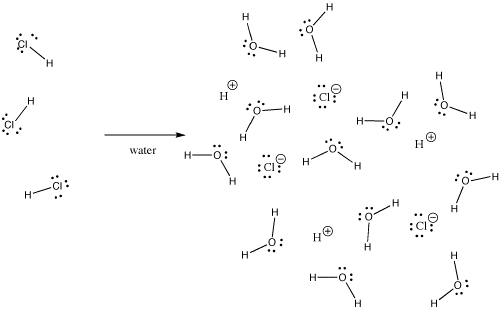
Figure IM7.2. HCl dissolving in water.
Potassium hydroxide, KOH, contains one bond that is covalent (O-H) and one that is ionic (K-O). Hydrogen is tricky because it is at the top of the periodic table as well as the left side. It is just electropositive enough to form ionic bonds in some cases. It is just electronegative enough to form covalent bonds in other cases.
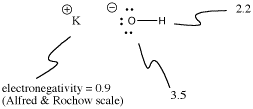
Figure IM7.3. Electronegativities and ionization in KOH.
In KOH, the K-O bond is ionic because the difference in electronegativity between potassium and oxygen is large. The difference in electronegativity between oxygen and hydrogen is not small. An O-H bond can sometimes ionize, but not in most cases. We think of the O-H bond as polar covalent rather than ionic.
Problem IM7.1.
Predict the direction of polarity in a bond between the atoms in the following pairs:
a) sulfur-oxygen b) silicon-fluorine
c) hydrogen-sodium d) chlorine-aluminum
Because it is so common that an element from the extreme left hand of the periodic table is present as a cation, and that elements on the extreme right carry negative charge, we can often assume that a compound containing an example of each will have at least one ionic bond.
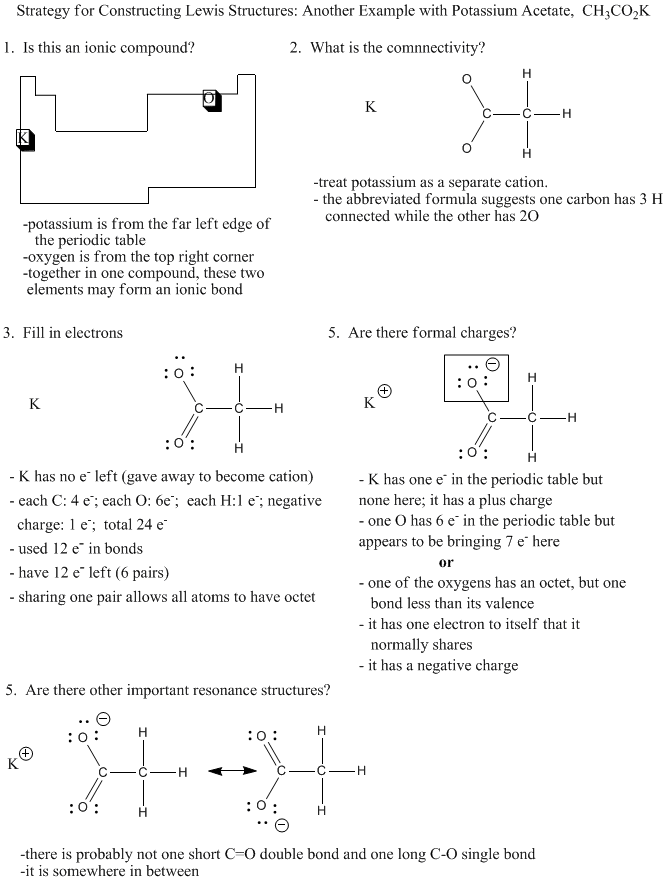
Figure IM7.4. An algorithm for constructing Lewis structures involving molecular ions.
Problem IM7.2.
Draw structures of the following compounds. Each one contains at least one anion and cation.
a) KBr b) LiOH c) KNO3 d) MgSO4 e) Na3PO4 f) Na2SO3
g) LiClO4 h) NaClO3 i) KNO2 j) Ca(ClO2)2 k) Ca2SiO4 l) Na3PO3
m) NaOCl n) Mg2SnO4
Problem IM7.3.
Ammonium ion, NH4+, is a common molecular ion. Draw structures for the following compounds that include this ion.
a) NH4Cl b) (NH4)2CO3 c) (NH4)3PO3 d) NH4CH3CO2 e) NH4HSO4
Problem IM7.4.
Many anions have names that tell you something about their structure.
| prefix- or -suffix |
common meaning |
example name | example formula |
| -ide | atom is present as anion | chloride | Cl - |
| -ate | atom is present as an oxyanion; usually a common form | chlorate | ClO3- |
| -ite | atom is present as an oxyanion, but with fewer oxygens (or lower "oxidation state") than another common form | chlorite | ClO2- |
| per- | atom is present as an oxyanion, but with even more oxygens than the "-ate" form | perchlorate | ClO4- |
| hypo- | atom is present as an oxyanion, but with even fewer oxygens than the "-ite" form | hypochlorite | ClO- |
Using the table as a guide, propose names for the following anions:
a) Br- b) O2- c) F- d) CO32- (common oxyanion) e) NO3- (common oxyanion) f) NO2-
g) S2- h) SO42- (common oxyanion) i) SO32- j) SO52- k) C4- l) N3- m) As3-
n) PO43- (common oxyanion) o) PO33- p) I- q) IO3- (common oxyanion) r) IO4-
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure &
Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation:
Back to Structure & Reactivity Web Materials