Structure in Chemistry
Macromolecules
MM6. Polymer Synthesis
Macromolecules are very large molecules. Their molecular weights can range from the thousands to the millions. Typically they are constructed from small, repeating units linked together in some way.
How many repeating units are there in a chain? Because of the way synthetic polymers are made, we may be able to predict how long the chain is by the conditions we choose when we make it. Polymers are frequently made through the action of an initiator, which gets the monomer units to start attaching to each other. Without the initiator, the monomers just sit there. This method of synthesis is sometimes called "chain-growth". A reactive part of the growing chain keeps picking up monomers and adding them into the chain.
Not all polymers are made that way. Nylon 6-6 and other condensation polymers are sometimes made just by mixing two different kinds of monomers together, without an initiator. This method of synthesis is sometimes called "step growth". In step growth, all of the monomers are ready to react with each other, and they all progress at the same time, in step with each other.
For now, we are going to focus on chain growth because there are some specific features of this process that are useful to know about, and they might be a little less intuitive than what happens with step growth. In chain growth, if each initiator in the process starts its own chain growing, then the ratio of monomers to intiators that we start with may tell us the chain length we will end up with. We refer to that ratio as the feed ratio. If we start with ten moles of monomer and one mole of initiator, the feed ratio is 10. Once that initiator starts a chain growing, there should be nine monomers left, and we would predict the chain would be ten units long. But if we start with a hundred moles of monomer and one mole of initiator, then as soon as it starts growing the growing chain would have ninety nine more monomers it could enchain, and we would predict it could make a polymer one hundred units long.
But how do we know if that really happens? We need some kind of experiment to measure how long the polymer chain really is if we want to know if the prediction came true. There are a number of ways to do that measurement.

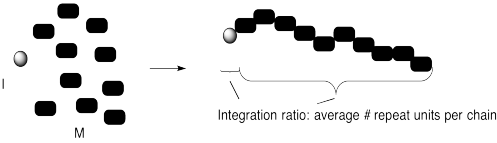
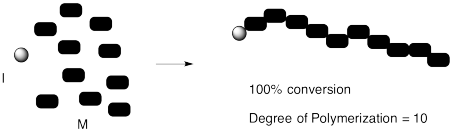
We can confirm that outcome experimentally by measuring the ratio of monomer to initiator in the polymer that is produced. That evidence is sometimes obtained by integration of a 1H NMR spectrum. The "degree of polymerization" is the measured number of repeating units in the chain. In the case below, we started with 10 monomers per initiator and ended up with DP = 10.
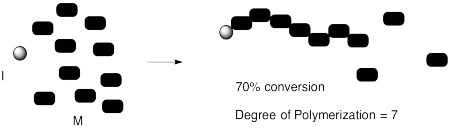
Things don't always work out that way. Maybe for some reason the chain stops growing unexpectedly (an event that is often called "chain death"). Maybe we stopped the reaction a little too early, before all the monomers had time to react. Maybe there is a reverse reaction, in which a polymer can break down into monomers again, and we end up at some balance between polymer formation and polymer breakdown (an "equilibrium").
In that case, the degree of polymerization might not reflect the original monomer to initiator ratio. It might also depend upon the percent conversion -- that is, how much of the original monomers were converted into polymer. If we start with 10 monomers per initiator, but only 70% of the monomers are enchained in the reaction, then the degree of polymerization will be 7, not 10.
If we are dealing with a block copolymer, we may be interested in more than just the total number of monomers in the chain. We may instead be interested in the ratio of one monomer to the other (for example, A:B:A = 6:6:6 in the example below).
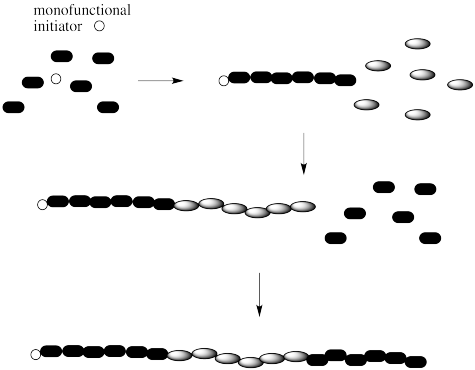
Note that there are a couple of different ways of making block copolymers. We can simply start growing the chain from one end, as shown above. Alternatively, we might start with a difunctional initiator. That's an initiator that starts gorwing the chain in two directions at once. The result is called a "telechelic polymer". This method of growth can be more efficient in some cases.
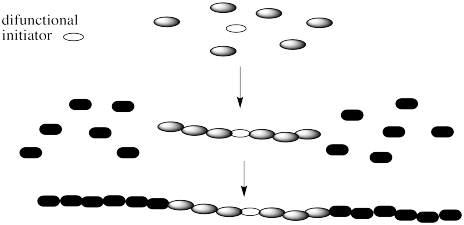
Problem MM6.1.
Explain why a telechelic polymer might grow more quickly than a regular one.
Problem MM6.2.
If a polymerization is performed using a 500:1 ratio of initiators to monomers and the reaction reaches 85% conversion, what is the degree of polymerization?
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation: