IC. Ionic Compounds
IC4. Solubility
One of the other general properties of ionic compounds is that they often have some solubility in water. The oceans, of course, are saltwater. In a mixture, two or more materials are mixed together but they remain essentially separate, like sand and water. You can still easily tell the difference between the sand and the water, because even if you shake them up they will separate again on their own.
In a mixture, two or more materials are mixed together but they remain essentially separate, like sand and water. You can still easily tell the difference between the sand and the water, because even if you shake them up they will separate again on their own.
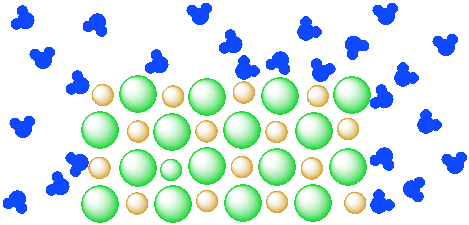
Figure IC4.1. A mixture of an insoluble salt (orange and green ions) and water (blue molecules). The two components remain separate from each other.
In a suspension, one or more materials is mixed into a liquid, and the mixture becomes somewhat homogeneous. Instead of having easily identifiable layers, the liquid looks the same throughout. However, suspensions are generally cloudy liquids. Milk is a suspension. It contains water, fats and proteins. They may settle out into separate layers eventually, but it takes time.
In a solution, one or more materials is mixed into a liquid, and the mixture becomes a completely homogeneous liquid. Solutions are transparent, not cloudy. They may be coloured or colourless, but you can always see through them. Saltwater is a solution.
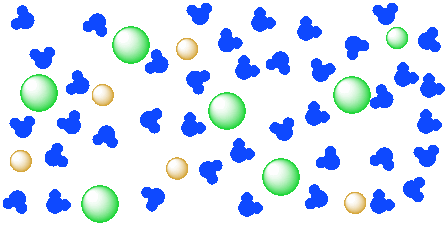
Figure IC4.2. A solution of a salt (orange and green ions) in water (blue molecules). The ions of the salt are completely distributed throughout the water.
You can't see chunks of salt in the solution because the salt particles are too small for you to see. The salt is separated into individual ions, surrounded by water molecules.
Of course, if you put some salt in water, it might not dissolve right away. You might have to stir it for a while.
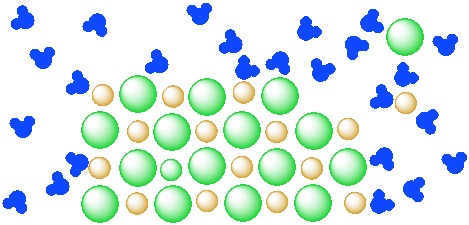
Figure IC4.3. A mixture of a salt (orange and green ions) and water (blue molecules). The salt is beginning to dissolve in the water.
Eventually more of the salt would dissolve in the water. Additional ions would be pulled out into the water to become surrounded by water molecules.
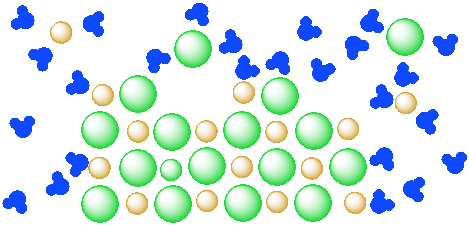
Figure IC4.4. A mixture of a salt (orange and green ions) and water (blue molecules). The salt continues to dissolve in the water.
However, at some point, the system might come to "equilibrium": the water has dissolved all of the salt that it can, so the rest of the salt stays solid. This equilibrium may be "dynamic": different ions may become dissolved in the water while others are deposited from solution into the solid state. However, the overall ratio of dissolved ions to water stays the same.
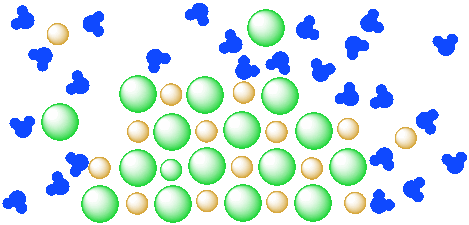
Figure IC4.3. A mixture of a salt (orange and green ions) and water (blue molecules). The salt is partly dissolved in the water but has reached equilibrium.
Problem IC4.1.
Let's take a look at the idea that a given amount of water is only able to dissolve a specific amount of salt.
a) In the diagram above, how many water molecules are there?
b) How many units of salt (an anion and a cation) are dissolved?
c) If there were only a dozen water molecules present, how many units of salt would dissolve?
d) If a hundred water molecules were present, how many units of salt would dissolve?
Why do salts dissolve in water? Water is a molecular compound; the atoms are directly attached to each other, rather than being ions that are attracted to each other. Because of electronegativity differences, the oxygen in water has a partial negative charge and the hydrogens have partial positive charges. Ionic compounds can dissolve in polar liquids like water because the ions are attracted to either the positive or negative part of the molecule.
Note that there is a sort of tug-of-war involved when things dissolve in water. The water is pulling individual ions away from the solid. The solid is pulling individual ions back out of the water. There exists an equilibrium at some point, based on how strongly the water attracts the ions, versus how strong the ionic solid attracts the ions.
You might expect to be able to predict vaying degrees of solubility in water for different ionic compounds. You would just use the principles of Coulomb's law that we used in melting points. The smaller the ions, the closer together they would be, and the harder it would be for the water molecules to pull the ions away from each other.
Problem IC4.2.
Predict which of the following pairs should be more soluble in water, based on what you know about Coulombic attraction between ions.
a) LiF or NaF
b) NaK or KF
c) BeO or LiF
Problem IC4.3.
Although lithium fluoride and magnesium oxide contain cations and anions of roughly the same size, lithium fluoride is much more soluble in water (2.7 g/L) than magnesium oxide (0.087 g/L) at room temperature. Propose a reason why.
However, the trends we saw in melting points in ionic compounds become more complicated when it comes to solubility. The water solubility of alkali chlorides does not follow a simple trend (Table IC4.1).
Table IC4.1 Water solubility among alkali chlorides.
