Structure & Reactivity in Chemistry
Structure-Property Relationships
SP11. Hydrogen Bond Acceptors
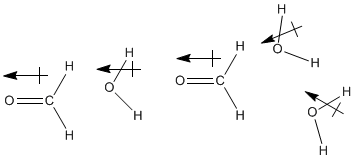
Formaldehyde is another example of a compound that dissolves well in water, and in fact the most common way to obtain formaldehyde is as an aqueous solution. You may have encountered such a solution in an anatomy or general biology lab, because formaldehyde solutions are used as preservatives for biological specimens. Of course, water and formaldehyde are both polar molecules, so it is easy to imagine their dipoles interacting together. However, formaldehyde does not have very strong hydrogen bonds like water does, so at first glance it seems as if formaldehyde might not interact with water molecules as strongly as water molecules interact with each other.

Figure SP11.1. Dipoles aligning between methanal and water.
Actually, the interaction between these molecules may be stronger than it first seems. Although formaldehyde doesn't engage in strong hydrogen bonds by itself, in the presence of water or another protic compound -- one that contains a very positive hydrogen, such as H2O, HF or NH3 -- strong hydrogen bonds do form.
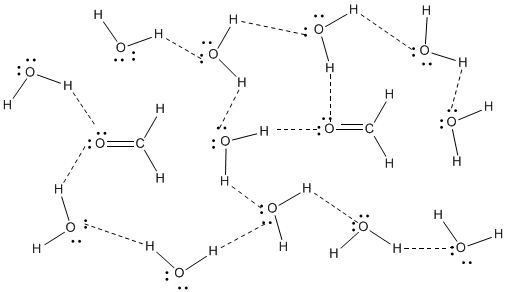
Figure SP11.2. Hydrogen bonding between methanal and water.
This occurs because formaldehyde has an oxygen atom with lone pairs and so it can act as a hydrogen bond acceptor. That means it can engage in hydrogen bonding with something that does have positive hydrogens that can interact with its lone pairs. The water in this case is acting as the hydrogen bond donor for formaldehyde. Hydrogen bond acceptors are often important in biological systems, where nearly everything takes place in the presence of water.
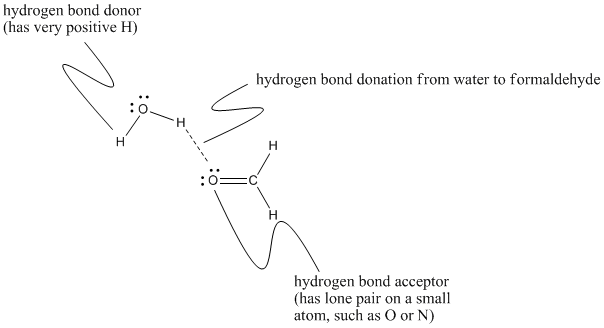
Figure SP11.3. Different contributions to hydrogen bonding between methanal and water.
The language used here can sound a little bit backwards if you are familar with Lewis acid-base concepts. We often think of strong interactions between molecules coming about because a source of electron density (such as a lone pair) is attracted to an electron-deficient atom (such as a partially positive proton). In that case, we describe one partner as the electron donor and the other one as the electron acceptor. The names "hydrogen bond acceptor" and "hydrogen bond donor" are more closely related to the way we talk about proton transfers between acidic and basic molecules. Even though the fundamental interactions are the same as in Lewis acid-base interactions, we sometimes describe them in the reverse way: a proton donor donates a proton to an electron-rich proton acceptor.
Problem SP10.1.
Which of the following compounds are capable of hydrogen bonding? Which are hydrogen bond acceptors? Which are neither?
a) triethylamine b) pentanal c) ethylamine d) diethyl ether e) butanamide f) hexene
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.
 Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation: