Structure & Reactivity in Chemistry
Structure-Property Relationships
SP3. Kinetic-molecular theory
If you know about ideal gases, you know that their behavious is easily predicted regardless of its identity. Whether the gas is helium or neon, if we change the pressure of the gas then we know exactly how its volume will change. Similarly, if we change the temperature of a gas, we know exactly how its volume will change.
In part, these straightforward relationships are explained by the kinetic molecular theory of gases. This model assumes gas molecules or atoms are like little balls that zip along in straight lines until they hit something and bounce off. That doesn't happen very often, because there is a lot of room between the particles, but it will happen more often if the volume decreases and the particles have less room. In that case, the pressure increases because of an increasing nuber of collisions between the particles and their container. The relationship works two ways. If pressure is exerted in a gas from the outside, squeezing it into a smaller space, then the particles will collide more often. Conversely, if the molecules are made to speed up and collide more frequently, they will exert greater pressure on their surroundings.
But there is another important aspect of this model, and that is the assumption that the kinetic energy of a gas is proportional to its temperature in Kelvin. It doesn't matter whether the gas is helium or neon, but at 375 K the kinetic energy of each is the same. Temperature is therefore an indicator of how much energy is available. The higher the temperature, the more energy there is available, and the faster molecules can go.
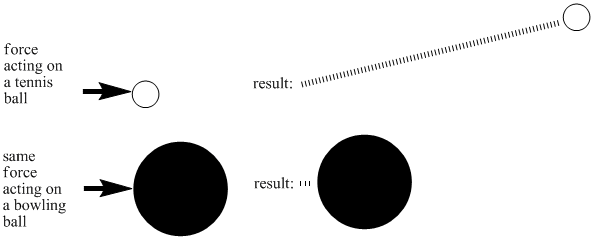
Different kinds of matter require different amounts of energy to get moving. That's why some compounds are gases at room temperature while others are solids. There are many reasons for these differences. One factor is the mass of a molecule. If you hit a tennis ball with a tennis racket, you can be sure that it will sail through the air, but the same thing might not be true if you hit a bowling ball with a tennis racket. By putting an equal amount of energy into a tennis ball and a bowling ball, you would make the tennis ball move much faster than the bowling ball. Lighter objects need less energy to reach high speeds.

Figure SP3.1. The relationship between mass and inertia: more massive (heavier objects) take more energy to move.
A very similar relationship is true with gases. A helium atom and a neon atom both have the same amount of kinetic energy at 375 K, but the helium atom is moving much more quickly than the neon atom. That's because kinetic energy is proportional to both mass and speed of an object. If the mass gets greater, the speed must be lower in order to keep the kinetic energy the same.
For a molecular example, let's look at halogens. All the elemental halogens are simple diatomic molecules. Their chemical properties have many similarities, but they have very different boiling points and melting points. Iodine, with the greatest mass, is a solid at room temperature, while fluorine has the lowest mass and is a gas at pretty much all the temperatures you are likely to encounter on Earth.
Table SP3.1. Properties of the halogens in their elemental states.
| Name & formula | MW, g/mol | mp, ° C | bp, ° C |
| fluorine, F2 | 38.0 | -219.6 | -188.4 |
| chlorine, Cl2 | 70.9 | -100.98 | -34.6 |
| bromine, Br2 | 159.8 | -7.2 | 58.8 |
| iodine, I2 | 253.8 | 113.5 | 184.3 |
Clearly the mass of a molecule influences its state. Does the mass of a molecule alone allow us to predict its behavior? No. A second important factor involves another difference between gases, liquids and solids: attraction between molecules. The molecules in solids are not just sitting still; they are sitting still together. Getting a crystal of iodine to evaporate requires coaxing molecules away from each other, and overcoming this attraction between them.
In fact, intermolecular forces can even be important in the gas phase. The fact that some gas molecules can cling to each other, even just a little bit, leads to what is called "non-ideal behaviour". These kinds of gases do not quite follow ideal gas laws perfectly.
There are many different types of intermolecular attractions. The halogens in the table above cling to each other via the weakest type: London dispersion interactions. We will start by looking at those interactions, and then move to stronger kinds, such as dipole interactions and hydrogen bonding.
Problem SP3.1.
Based on the data in the table above, what would you predict for the melting point and boiling point of nitrogen, N2, and oxygen, O2?
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.
 Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation: