SImple heteroatomic functional groups contain atoms other than carbon and hydrogen. By far, the most common examples are alcohols, amines and ethers. Alcohols are organic compounds containing simple OH groups. Amines contain singly-bonded nitrogen atoms; the nitrogen may be connected to either carbon or hydrogen. Ethers contain singly-bonded oxygen atoms connected only to carbons.
It is useful to know the names of the normal (straight-chain) hydrocarbons, in which straight chains of hydrocarbons are connected by single bonds and the carbons' valences are saturated with hydrogens. The names of these compounds form the root names of other compounds having the same number of carbon atoms in a continuous chain.
| Number of carbons | Name |
| 1 | meth |
| 2 | eth |
| 3 | prop |
| 4 | but |
| 5 | pent |
| 6 | hex |
| 7 | hept |
| 8 | oct |
| 9 | non |
| 10 | dec |
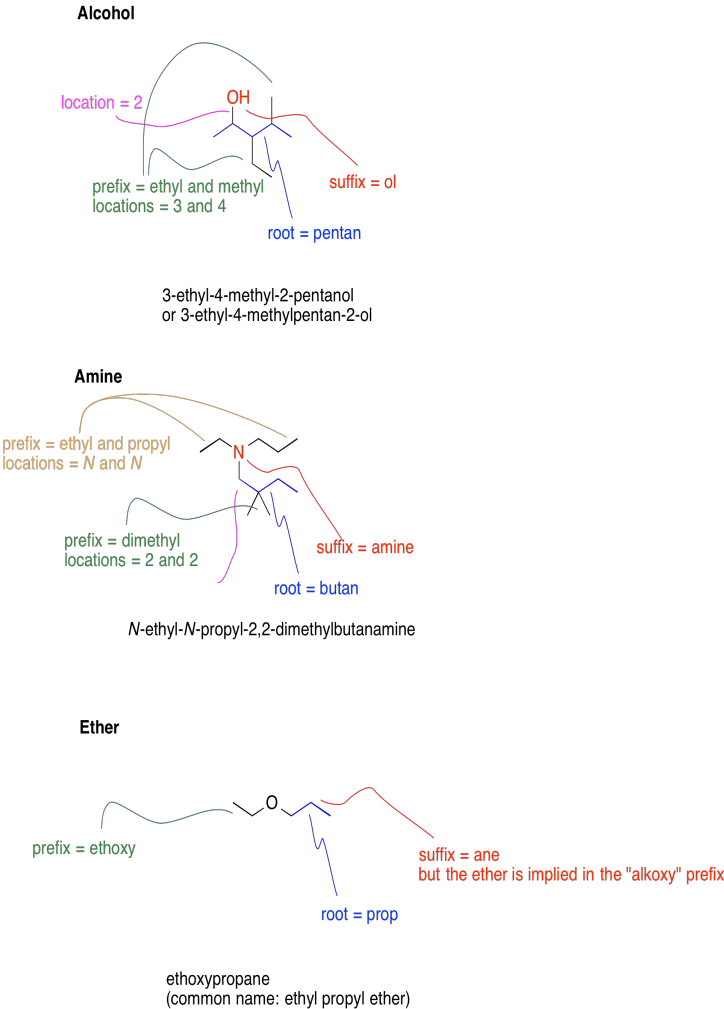
The names of these compounds are based on the names of alkanes, but the suffix of the name varies to indicate the functional group present.
| Class | Icon | Description | Modified prefix | Suffix |
| Alcohol | C-OH | contains OH connected to carbon | - | ol |
| Amine | C-NR2 | contains a nitrogen connected to carbon | N-alkyl | amine |
| Ether | C-O-C | contains a carbon-oxygen-carbon linkage | alkoxy | ane |
Note that, in formal naming, amines and ether may contain modified prefixes. In amines, the prefix N-alkyl, such as N-methyl or N-propyl, denotes an additional carbon chain is attached to the nitrogen, as well as the chain that forms the root name of the compound.
In ethers, the prefix alkoxy, such as ethoxy or butoxy, denotes the smaller chain that is attached to the main chain via an oxygen atoms. Note that ethers also have common names, in which the two chains are named as substituents in alphabetical order and the word "ether" is appended. For example, methoxypropane would also be called methyl propyl ether.
The following scheme provides representative examples of alcohols, amine and ethers as well as a brief guide to how the formal names of these compounds are put together based on their structures. Note that the root name is based on the longest continuous chain that contains the functional group. Substituents are named according to how many carbons they contain in a straight chain, and numbered based on where they are found along the main chain, in which each carbon can be numbered from one end to the other. The main functional group is present is always given the lowest possible number. If more than one functional group is present, the one that contains the most bonds to oxygen usually gets priority.

Note that, if more than one of the same type of substituent are present, a prefix is used to tell how many of them are present.
| Number | Prefix |
| 2 | di |
| 3 | tri |
| 4 | tetra |
| 5 | penta |
| 6 | hexa |
| 7 | hepta |
Problem FG2.1.
Provide structures for the following alcohols.
a) 1-propanol b) 3-heptanol c) 3-hexanol
d) 6-methylheptan-3-ol e) 5-ethylheptan-3-ol f) 2,4-dimethylpentan-3-ol
g) 6-ethyl-4-propyloctan-2-ol h) 2,2,3-trimethylpentan-3-ol i) 2-methyloctan-2-ol
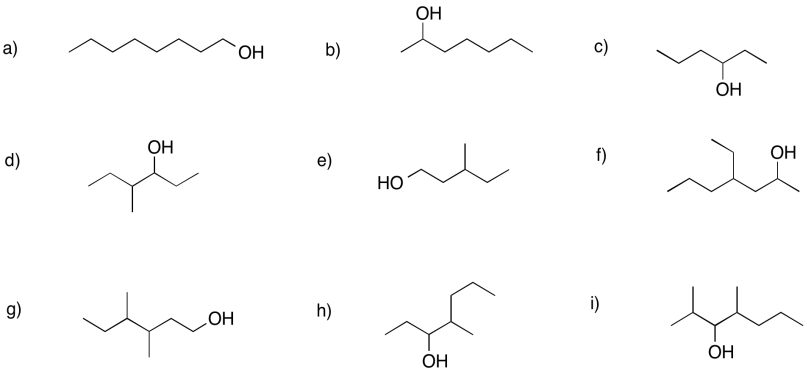
Problem FG2.2.
Provide names for the following alcohols.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by
Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.Navigation:
Back to Functional Group Appendix
Back to Web Materials on Structure & Reactivity in Chemistry