a) 1, 2, 3, 4, 5... a series of whole numbers, or n.
b) 2, 4, 6, 8, 10... a series of even numbers, or 2n (because every even number is two times another number).
c) 3, 5, 7, 9, 11... a series of odd numbers, or 2n + 1 (because every odd number is one more than some even number).
d) 1, 4, 9, 16, 25... a series of squares, or n2.
e) 2, 4, 8, 16, 32... a series in which each numer is double the last number, or 2n.
f) 1, 1/2, 1/4, 1/9, 1/16... a series of reciprocals of squares, or 1/n2.
Problem AT2.8.
a) When q increases, F increases. The increase is linear: if q1 doubles, F doubles. As the charge in the nucleus gets larger, the force of attraction gets larger.
b) When r increases, F decreases. The decrease is nonlinear: if r doubles, F drops by a factor of four, rather than a factor of two. As the distance from the nucleus gets longer, the attraction to the nucleus drops sharply.
c) Hydrogen and helium are both in the first row of the periodic table. To a rough approximation, the distance between nucleus and electron is similar in these two atoms. However, helium has a charge of 2+ in its nucleas, compared to the 1+ charge in the nucleus of a hydrogen atom. As a result, the attraction of an electron on helium to the nucleus would be about twice as great as the attraction of an electron on hydrogen to its nucleus.
Helium's electrons are much more tightly held than hydrogen's.
The situation is really much more complicated than that. For example, if helium's electrons are more tightly attracted to the nucleus than hydrogen's, then helium's electrons ought to be pulled closer to the nucleus than hydrogen's. That means helium's electrons are held even more tightly than we at first thought.
Another complicating factor is that helium has two electrons, whereas hydrogen has only one. An electron may be attracted to the nucleus, but electrons repel each other. That second electron in helium should offset the extra attraction to helium's more positive nucleus. That means helium's electrons may be less tightly attracted than we originally thought.
However, the effect of the second electron is much smaller than it first appears. That's because the second electron could be anywhere around the helium atom. It has a 50% chance of being further away from the first electron than that positively charged nucleus. The farther it is away, the smaller its influence. Helium's electrons are definitely more strongly attracted to the nucleus than are hydrogen's, but it is difficult to say exactly how much more without the help of some more sophisticated tools.
Problem AT2.9.
a) As wavelength gets longer (value of λ increases), energy decreases.
b) As frequency gets higher (value of ν increases), energy increases.
Problem AT2.10.
| Element Symbol | Atomic Number | Mass Number | Number of Protons | Number of Neutrons | Number of Electrons | Charge |
| H | 1 | 1 | 1 | 0 | 1 | 0 |
| H | 1 | 1 | 1 | 0 | 0 | +1 |
| H | 1 | 1 | 1 | 0 | 2 | -1 |
| H | 1 | 2 | 1 | 1 | 1 | 0 |
| H | 1 | 3 | 1 | 2 | 1 | 0 |
| Be | 4 | 9 | 4 | 5 | 2 | +2 |
| C | 6 | 12 | 6 | 6 | 6 | 0 |
| Mg | 12 | 25 | 12 | 13 | 12 | 0 |
| Tc | 43 | 98 | 43 | 55 | 43 | 0 |
| Ca | 20 | 40 | 20 | 20 | 18 | +2 |
| Si | 14 | 28 | 14 | 14 | 14 | 0 |
| K | 19 | 47 | 19 | 28 | 15 | +4 |
| Fe | 26 | 56 | 26 | 30 | 23 | +3 |
| Br | 35 | 79 | 35 | 44 | 36 | -1 |
| Ti | 22 | 39 | 22 | 17 | 21 | +1 |
| P | 15 | 30 | 15 | 15 | 15 | 0 |
| Al | 13 | 27 | 13 | 14 | 10 | +3 |
| S | 16 | 32 | 16 | 16 | 16 | 0 |
| Pd | 46 | 106 | 46 | 60 | 45 | +1 |
| Cr | 24 | 52 | 24 | 28 | 21 | +3 |
| Sn | 50 | 118 | 50 | 68 | 50 | 0 |
| Hg | 80 | 200 | 80 | 120 | 79 | +1 |
| Au | 79 | 197 | 79 | 118 | 78 | +1 |
Problem AT3.1.
a) The energy of the electron gets higher.
b) The energy of the electron gets lower.
c) The wavelength gets shorter.
d) The wavelength gets longer.
Problem AT3.2.
The probability of finding an electron at a node is zero.
Problem AT3.3.
Your drawing is beautiful. You should put it on your fridge, or else send it to your mother.
Problem AT4.1.
a) 2s b) 3p c) 3d e) 5f e) 4s
Problem AT4.2.
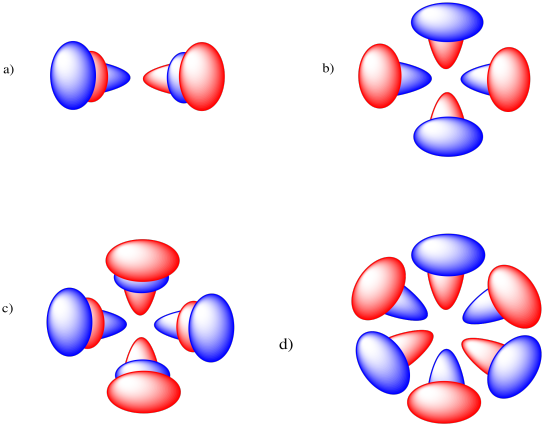
Problem AT4.3.
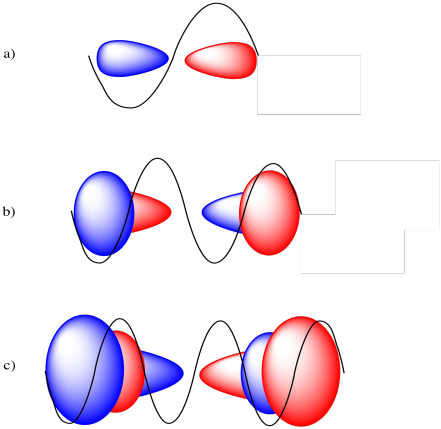
From one end of the orbital to another, the 2p orbital covers a full sine wave, a 3p orbital covers two full sine waves, and a 4p orbital covers three full sine waves.
Problem AT5.1.
a) O: 1s22s22px22py12pz1
b) S: 1s22s22p63s23px23px23py13pz1
c) Si: 1s22s22p63s23px23px13py1
d) N: 1s22s22px12py12pz1
e) Ar: 1s22s22p63s23p6
f) Ne: 1s22s22p6
Problem AT6.1.
a) Cl: [Ne]3s23px23py23pz1
b) Ca: [Ar]4s2
c) Al: [Ne]3s23px1
d) P: [Ne]3s23px13py13pz1
Problem AT6.2.
a) iron, Fe: [Ar]4s23d6
b) nickel, Ni: [Ar]4s23d8
c) mercury, Hg: [Xe]6s24f145d10
d) lead, Pb: [Xe]6s24f145d106p2
e) arsenic, As: [Ar]4s23d104p3
f) titanium, Ti: [Ar]4s23d2
Problem AT7.1.
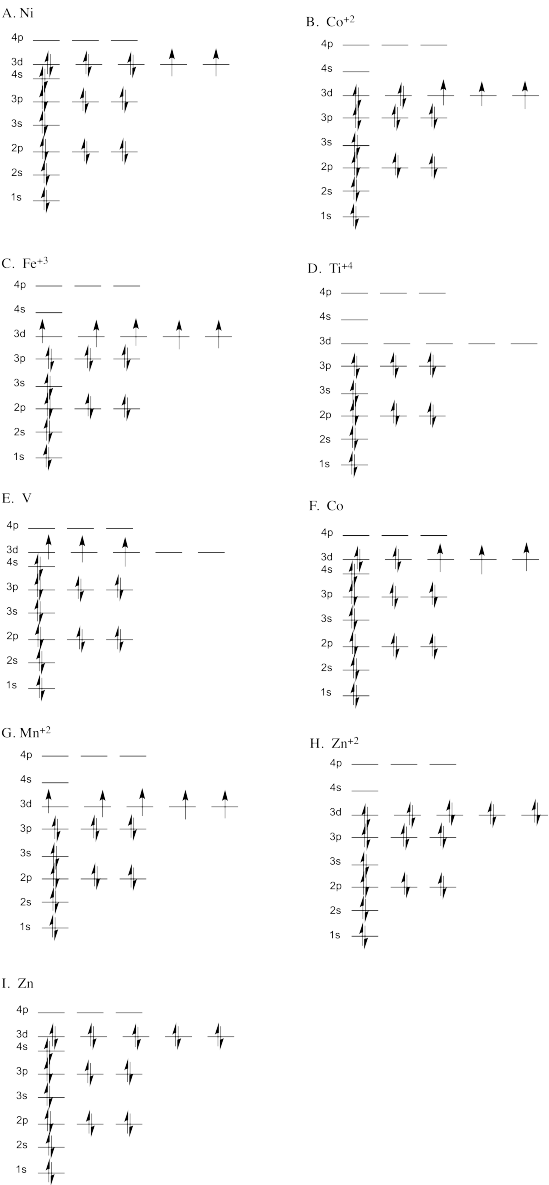
Problem AT8.1.
a) Electrons have occupied the 2p level before the 2s level is filled. The energy gap between 2s and 2p is greater than the energy cost to pair two electrons in the same orbital.
b) Two electrons have occupied the same orbital in the 2p level when there is another empty orbital. It costs additional energy to pair two electrons in the same orbital, but it would not cost additional energy to put a second electron in another orbital at the same energy.
c) Two electrons in two different orbitals in the 2p level are spin-paired. Energy can be lowered by keeping electrons in different orbitals parallel, increasing multiplicity.
d) The 2p level is fully occupied before the 2s level has been occupied. Electrons will generally go to the lowest possible energy level.
e) Two parallel electrons occupy the 2s level. These electrons have identical quantum numbers, which is not allowed by quantum mechanics. Electrons can only occupy the same orbital if they are spin-paired.
Problem AT9.1.
a) Zn: Cd, Hg
b) Ca: Mg, Ba
c) O: S, Se
d) Cl: F, Br
e) Cr: Mo, W
Problem AT9.2.
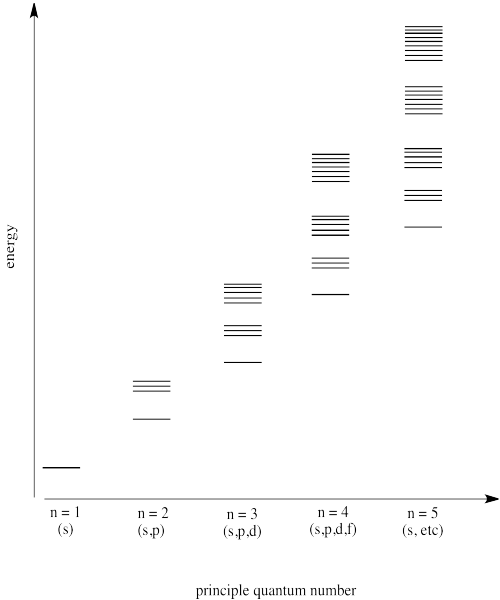
Problem AT9.3.
The atomic number is the number of protons in the nucleus. For two atoms in the same row of the periodic table, the outermost electrons are roughly the same distance away from the nucelus. The more positive protons there are in the nucleus, the more tighly held are the electrons.
Problem AT9.4.
Take it from the boron. The oxygen atom is holding its electrons much more tightly.
Problem AT9.5.
According to the drawing, the neon would take the electron, because of all the atoms depicted in the graph, neon attracts electrons most strongly. However, there is a complication. Although neon strongly attracts its own electrons, it can't accommodate an extra electron as easily as could fluorine, the next-best candidate. In "Lewis" terms, neon has a "full octet". In quantum terms, an additional electron would have a higher principle quantum number and be placed in the next "shell", farther from the nucleus. With spin-pairing, fluorine can accept another electron into its valence shell.
Problem AT9.6.
The oxygen would pull the electrons in the bond more tightly to itself.
Problem AT9.7.
Moving from one row to the next in the periodic table signifies that the outermost electron is in a shell farther from the nucleus. Those outermost electrons are less tightly held if they are farther from the nucleus.
Problem AT9.8.
a) Cs+ F-
b) Na+ O-
c) K+ H-
Problem AT9.9.
The attraction for an electron falls off with 1/r2. As the value of r gets larger and larger, the quantity 1/r2 will begin to approach a limit (of zero). As a result, the difference between two successive values of 1/r2 in a series gets smaller and smaller.
Problem AT9.10.
a) Mg > Ca
b) Sn > Pb
c) Sb > Ag
d) As > Ga
e) Cu > W
f) S > Tl
Problem AT9.11.
a) The electron is held by its attraction to the nucleus.
b) As the number of protons in the nucleus increases, the electron becomes more tightly held, and harder to remove.
The relationship is similar to the one seen for electronegativity, and not coincidentally. Electronegativity can be calculated in a number of ways, but one of those ways uses the ionization energy as a factor.
Electronegativity is a calculated value, whereas ionization energy is an experimentally determined one. This difference brings up an important philosophical distinction. To a beginning student, an experimental value seems faulty, whereas a calculated value sounds good. To an experienced chemist, an experimental value is verifiable; it is real. In contrast, a calculated value is enhanced in prestige only if it can be shown to agree with experiment.
Problem AT9.12.
In order to escape the atom, an electron must gain energy. As shown in problem AT5.2., the 2p energy level is higher than the 2s energy level. That means a 2p electron already has more energy than a 2s electron. The 2p electron will not need as much additional energy in order to escape from the atom. As a result, boron's ionization energy is a little lower than beryllium's.
The additional protons in the nucleus of carbon and nitrogen more than make up for that effect.
In the case of oxygen, the next electron is just added to a 2p level, but in this case it must be paired with another electron in the same region of space (in the same "orbital"). The repulsion between these electrons, or "pairing energy", slightly destabilizes the oxygen, so less energy will be needed to remove an electron.
Once again, the continued addition of extra protons eventually compensates for this pairing effect.
Problem AT9.13.
As with electronegativity, ionization energy decreases as the distance to the nucleus increases because of the 1/r2 relationship in Coulomb's Law.
Problem AT9.14.
a) Energy is released because of the attraction of the free electron to a nucleus. The electron moves to lower energy as it becomes stabilized by its interaction with the nucleus.
b) The electron can get much closer to the hydrogen nucleus thanto the lithium nucleus, and so on. More energy will be released owing to the strong interaction between the electron and the hydrogen nucleus compared to the interaction between the electron and the lithium (or sodium or potassium...) nucleus.
Problem AT9.15.
a) The data zig-zags, but broadly speaking there is an increase in electron affinity as the number of protons in the nucleus increases.
b) Beryllium and neon have zero electron affinity because the next electron in each case would be added to a higher energy level. In the case of beryllium, the next electron would go into the 2p energy level. An electron added to neon would go into the 3s energy level.
c) Although the next electron added to nitrogen would be added to a 2p level, it would have to be paired in the same region of space as an electron that was already there. The pairing energy in this case must be enough to offset the attraction of the electron to the nucleus.
Problem AT9.16.
Perhaps the simplest explanation is this: The addition of 14 extra protons between lanthanum and hafnium, compared to between yttrium and zirconium, results in a considerable contraction of the atom. The electrons are pulled inward by the added positive charge in the nucleus. Thus, elements in the third row of the transition metal block are not as large as might otherwise be expected, and in some cases are even smaller than their precedent elements.
Note that this is not the only explanation. Relativistic effects are also believed to play a role in the behaviour of these massive elements. Einstein's theory of relativity states that objects get heavier the faster they move. The velocity of an electron can be shown to increase with the charge in the nucleus of the atom. Thus, atoms that have very high atomic numbers have very, very fast electrons, and consequently very heavy ones. These heavy electrons sink toward the heavy nucleus, and the atom shrinks further.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.
 Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.