AT6. The Aufbau Process and the Periodic Table
You may already know that electron configuration is the reason the periodic table works the way it does. Mendeleev and others noticed certain elements had very similar properties, and that's because they have very similar electron configurations. Lithium has configuration 1s22s1 and its alkali sister, sodium, has configuration 1s22s22px22py22pz23s1 . In both cases, the last electron added is an unpaired s electron. The last electron, or last few electrons, added to an atom generally play a strong role in how the atom behaves. This "frontier" electron is the one at the outer limits of the atom. If the atom is to interact with anything, the frontier electron will encounter the thing first. In contrast, the "core" electrons closer to the nucleus are more protected from the outside.
There are some shortcuts we take with electron configurations. We tend to abbreviate "filled shells" (meaning all the possible orbitals with a given principle quantum number are filled with electrons) and filled "sub-shells" (like 2s or 2p). First of all, in the case of p orbitals, if all the p orbitals are filled, we might just write 2p6 instead of 2px22py22pz2, because there is only one way all the orbitals could be filled. However, we wouldn't necessarily write 2p2 instead of 2px12py1, because we may wish to make clear that the configuration does not involve two electrons in one p orbital at that point, as in 2px22py0.
Also, we dispense with orbital labels entirely to ignore core electrons in a filled shell. For example, instead of writing 1s22s1 for lithium, we can write [He]2s1. Instead of writing 1s22s22px22py22pz23s1 for sodium, we write [Ne]3s1. The [He] means all the electrons found in a helium atom, which is a noble gas. The [Ne] means all the electrons found in a neon atom. A noble gas is an unreactive element with a filled shell: helium, neon, argon, krypton, xenon or radon.
[He] = 1s2 so [He]2s1 = 1s22s1
[Ne] = 1s22s22p6 so [Ne]3s1 = 1s22s22p63s1
The electrons beyond the noble gas shell are called the valence electrons. Lithium has one valence electron; it's the electron in the second shell. Sodium has one valence electron; it's the electron in the third shell. Neon has eight valence electrons; it's the entire second shell.
Problem AT6.1.
Write abbreviated electron configurations for the following elements.
a) chlorine, Cl
b) calcium, Ca
c) aluminum, Al
d) phosphorus, P
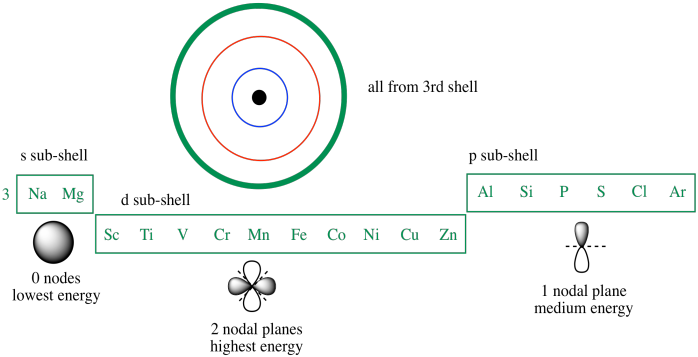
Principle quantum number 3 actually allows a third set of orbitals. These are called the d orbitals. The d orbitals are a little like p orbitals, but they are extend in two directions instead of just one direction. A d electron, for example, might extend along the x axis and the y axis, but not in between the axes. For comparison, a p orbital might extend along only one of these axes.
The d orbitals have five allowed orientations. They can be found along the x and y axes (called dxy), along the x and y axes (called dxz), or along the y and z axes (called dyz). Alternatively, they might be found in between the axes instead, rotated 45 degrees away from one of the other d orbitals. One of these, called the dx2-y2 orbital, is found between the x and y axes. In the same way, you could imagine an orbital between the x and z and between the y and z axes, but that would make six different orientations. Quantum mechanical rules don't allow that. As a result, two of the possible combinations collapse into a mathematical sum, making just one orbital. We call this one the dz2 orbital.
If we look at the third row in the periodic table, we see those three sub-shells (the s, then the p, then the d). Again, the 3s sub-shell fills first. With fewer nodes, this orbital is lower in energy than either the 3p or the 3d. The 3d orbital has more nodes than the 3p, so it is even higher in energy than the 3p. In fact, it is even a little higher in energy than the 4s orbital, the first sub-shell of the next shell.

Figure AT6.1. The third shell (n = 3) in the periodic table.
So, the 3d orbitals are higher in energy than the 3p orbitals. The 3d level is very similar in energy to the 4s level. In fact, the 3d energy level is just a little higher than the 4s energy level. For that reason, calcium's last electrons go into a 4s orbital, not a 3d orbital. Calcium behaves much like magnesium as a result.
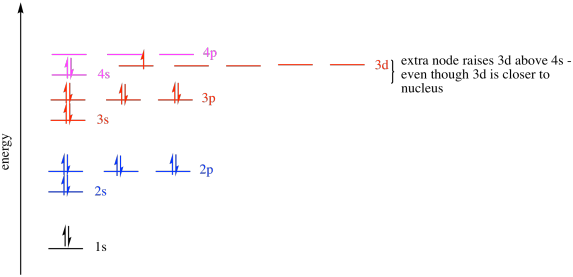
Figure AT6.2. Some of the additional energy levels available for electrons.
Problem AT6.2.
Write abbreviated electron configurations for the following elements.
a) iron, Fe
b) nickel, Ni
c) mercury, Hg
d) lead, Pb
e) arsenic, As
f) titanium, Ti
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.
 Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation: