TM2. Building Blocks: Metal Ions and Ligands in Transition Metal Complexes
Transition metal complexes or coordination complexes are molecules that contain groups arranged around a central metal ion. In a way, these are like "lego-molecules", easily assembled from smaller parts, and sometimes they are easily transformed into new molecules by switching out old parts for new ones. That rapid assembly and disassembly is part of what makes these comppounds very useful in both industrial and biological catalysis.
What are the building blocks that go into a coordination complex? The key, central part is usually a transition metal ion (although cations from other parts of the periodic table are also seen in some cases). The following table shows some common examples of ions formed by each of the transition metals.
Table TM2.1. Some common transition metal ions.
| Sc3+ | Ti4+ Ti3+ |
V5+ V4+ |
Cr3+ Cr6+ Cr2+ |
Mn2+ Mn4+ Mn7+ |
Fe3+ Fe2+ |
Co2+ Co3+ |
Ni2+ | Cu2+ Cu+ |
Zn2+ |
| Y3+ | Zr4+ | Nb5+ Nb3+ |
Mo6+ Mo4+ Mo5+ |
(Tc7+)* (Tc4+)* |
Ru3+ Ru4+ Ru2+ |
Rh3+ Rh+ |
Pd2+ Pd4+ |
Ag+ Ag2+ |
Cd2+ |
| La3+ | Hf4+ | Ta5+ | W6+ W4+ W5+ |
Re4+ Re6+ Re7+ |
Os4+ Os3+ |
Ir4+ Ir3+ |
Pt2+ Pt4+ |
Au3+ Au+ |
Hg2+ Hg1+ |
*radioactive, with a short half-life; seldom observed.
For each transition metal, the most common form of ion is listed first. For example, iron is often found in compounds as Fe3+. Other common ions are also shown below that; iron is seen pretty frequently as Fe2+. Other charges are possible; iron has been reported with charges all the way from Fe0 to Fe6+, but these cases are less common.
Also, it's worth noting that the top row of transition metals is generally much more common than the next two rows. That doesn't mean that they are less important; gold (Au), silver (Ag) and platinum (Pt) are certainly important economically. However, you may be more likely to encounter examples of complexes from the first row. That's especially true in biological chemistry, because organisms have evolved to make use of those metal ions that are most readily available to them.
Problem TM2.1.
a) The metals on the left hand side of the table tend to have relatively high charges compared to the metals on the right. What do ions such as Sc3+, Zr4+, and Ta5+ have in common that would explain this trend?
b) The metals on the right side of the table have relatively low charges. In nature, metals such as copper (Cu), silver (Ag), and gold (Au) are frequently found as native metals (e.g. Cu0, with no charge at all) rather than as compounds. Explain this preference for low charges in this part of the transition metals.
c) The metals in the middle, on the other hand, have very wide ranges of charges (that's why the table lists three common charges for those, although even more exist). Why?
The charge on the metal ion is sometimes called the oxidation state. This term refers to the fact that metals that are exposed to the elements sometimes become positively charged, forming compounds such as metal oxides. The oxygen from the air provides the oxide or hydroxide ion to counter the charge when the metal atom loses electrons and becomes a metal cation. Familar examples include the oxidation of aluminum metal to form silvery-white aluminum oxide; you may have seen aluminum screen doors that are actually covered in a coating of aluminum oxide. The Statue of Liberty was originally covered in copper metal, but quickly became coated in money-green copper oxide.
Sometimes, within a complex, the charge or oxidation state of the metal is denoted using Roman numerals. For example, Co3+ is sometimes writted Co(III); Mn2+ might be written Mn(II).
So, if you need to brush up on Roman numerals:
1 = I; 2 = II; 3 = III; 4 = IV; 5 = V; 6 = VI; 7 = VII; 8 = VIII; 9 = IX
After that, we won't need to worry about it. Sometimes osmium is found as Os(VIII), for example, but we don't see a 9+ charge very often.
The metal ion is the first building block. The second building block is the ligand. The ligands are the pieces that are arranged around the metal ion. On the last page, we saw chloride anions (Cl-) and ammonia (NH3) act as ligands in different transition metal complexes. It is easy to imagine how a positively charged metal ion would attract a negatively charge anion such as chloride. As it happens, neutral ligands are just as common. The main requirement for sticking to a metal ion is a non-bonding pair of electrons, or a lone pair (at least, that's the case at this stage of your education).
The following table illustrates a variety of ligands for transition metal complexes.
Table TM2.2. Some common ligands.
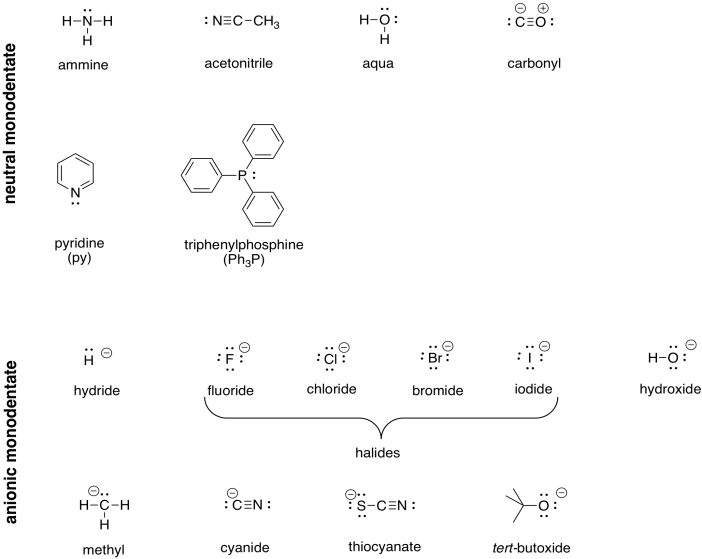
Problem TM2.2.
Calculate the overall charge on the following complexes, if any.
a) [Fe(II)(OH2)6] b) [Cr(III)(NH3)5Cl] c) [(py)4Mn(II)(SCN)2]
d) [Fe(II)(CN)6] e) [Co(III)(CN)5CO] f) [Fe(II)(CN)5NH3]
A coordination complex has a central metal ion with a number of ligands arranged around it. The last piece is the counterions, which would balance out the charge of the transition metal complex ion. We saw examples of common counterions on the introduction page.
Problem TM2.3.
Calculate the charge or oxidation state on the metal ion in each of the following complexes.
a) [Cr(OH2)6](NO3)3 b) K3[FeF6] c) [Cr(SCN)(NH3)5]SO4
d) K4[Mn(CN)6] e) [Au(NCCH3)2]ClO4 f) [(Ph3P)Ag(CN)]
On the next page, we will see how some ligands can bind to a metal more than once. That helps them hold on more tightly.
See a more in-depth discussion of coordination complexes in a later course.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.
 Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation: