TM5. Isomerism in Transition Metal Complexes
Sometimes, two compounds can have the same molecular formula but different structures. In transition metal complexes, that can happen if the same ligands are arranged spatially around the metal in different ways.
For example, the complex ion [CoCl2(NH3)4]+ contains two chlorine ligands that can be placed in two different ways around the cobalt. The cobalt in this complex has octahedral geometry. Every ligand in the complex is 90 degrees away from four other ligands, and 180 degrees away from a fifth. If there are two chloride ligands, then they might be found 90 degreed away from each other, or they might be found 180 degrees away from each other. The former case exists, and it is called the cis-isomer; the latter case also exists, and is called the trans-isomer (cis means same, as in same side, as opposed to trans, which means opposite). These two isomers are examples of geometric isomers, two compounds in which the same parts are found at different angles to each other.
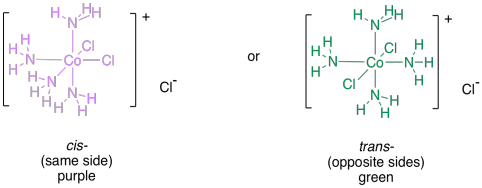
Figure TM5.1. Geometric isomers in coordination complexes.
In this case, the two compounds have different physical properties, most readily seen in the two different colours of the complexes: one is purple and the other is green. The exact reasons for the colours are subtle; you're not expected to know why one is green and the other is purple at this stage.
Problem TM5.1.
Draw structures for the following compounds:
a) trans-Fe(py)4Cl2 b) trans-Mn(OH2)4F2 c) cis-(bpy)V(acac)Cl2 d) cis-Mo(CO)4(PMe3)2
A group of three ligands can also adopt two different geometries in an octahedron. The three ligands can be found clustered together on one side of the complex. They occupy one face of the octahedron, in what is called the fac-geometry. Alternatively, they could lie all in a row. Sitting along a meridian of the octahedron, they adopt a mer-geometry.
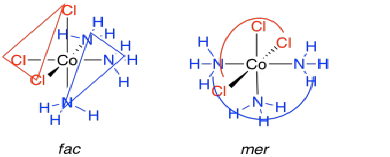
Figure TM5.2. Fac and mer isomers in coordination complexes.
Problem TM5.2.
Draw structures of the following complexes:
a) fac-W(CO)3(NCCH3)3 b) mer-Mo(CO)3(PMe3)3 c) fac-Mo(CO)3(PF3)3
d) mer-Mo(CO)3(PCl3)3 e) fac-Na[Mn(CO)3(CN)3]
See a more in-depth discussion of coordination complexes in a later course.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.
Send corrections to cschaller@csbsju.edu Navigation: Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.