Monomers & Polymers
MP8. Polymer Topology
Polymers are very large molecules made from smaller ones. How those smaller units are arranged within the polymer is an issue we haven't addressed very closely yet. Topology is the study of three-dimensional shapes and relationships, or of how individual parts are arranged within a whole. Let's take a look at the topology of polymers.
At the simplest level, we have been thinking of polymers as chains of monomers strung together like beads on a string. We even use the verb "enchain" to describe the act of taking a monomer and tying it into a larger polymer. The chain is the most basic of polymer structures. This topology is often refered to simply as a chain or, to underscore the structure, a linear polymer.

Even a simple chain, in this context, can have additional structural features worth considering. These features are connected to how a polymer chain grows up from individual monomers. If the polymer results from a chain reaction, typically used for the polymerization of alkenes, then the growing chain usually has two distinct ends. One end, sometimes called the tail, is the site of the first monomer to be incorporated into the polymer, as well as some remnant of the initiator to begin the polymerization process. The other end, sometimes called the head but more commonly just the growing end, is the active site, the place where new monomers are about to be enchained into the polymer.
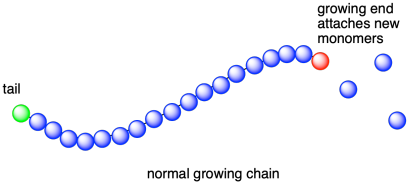
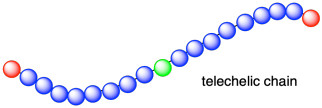
That's what happens in most cases, when the initiator is capable of reacting with one monomer and starting the chain reaction. However, sometimes initiators are difunctional. Difunctional initiators are capable of getting two monomers to start growing a polymer chain: one in each direction. In that case, the initiator fragmentis left behind in the middle of this new growing chain, which grows outward from the middles. Both ends of the chain are growing ends. In this type of growth, the chain is refered to as "telechelic".
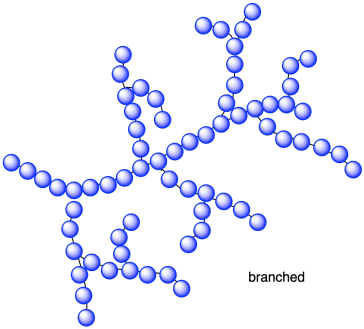
Sometimes, polymer chains do not have simple, linear structures. Instead, their chains branch out here and there. This topology is caled "branched". Instead of looking like a snake or a piece of spaghetti, this structure looks more like a length of seaweed. Branching is sometimes an artifact of how the polymer was made, and so sometimes the same monomers may lead to a more linear polymer or a more branched one. The prime example if polyethylene, which can form high-density polyethylene (HDPE) or low-density polyethylene (LDPE) depending on the conditions under which the ethylene is enchained.
In a branched polymer, smaller chains grow out like limbs along a tree trunk or leaves along a stem. We can take that arrangement a step further into something that looks more like a net. In what we call a crosslinked structure, branches connect one main chain to the next, tying them together into one big piece. Sometimes, this type of structure is called a thermoset.
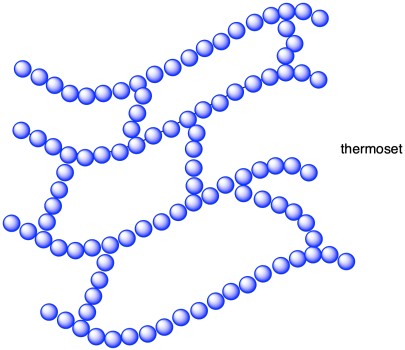
The word "thermoset" is really a description of a physical property of a polymer, in contrast to "thermoplastic". A thermoplastic is a polymer that can be melted and reformed into new shapes after polymerization. In contrast, once a thermoset has been polymerized, it retains its shape even when heated; it doesn't melt. These terms have connotations about the topology of the material, nonetheless.
The reason thermoplastics can be melted and formed into new shapes is that they are made of separate molecules. The molecules may be very long, and they may even be branched, but at high enough temperatures these molecules can move completely independently of each other. They can melt and so the material that is comprised of these separate molecules take on new shapes.
In a thermoset, crosslinks connect the different chains in the material, forming bridges that span from chain to chain to chain, essentially uniting the material into one big molecule. If it is one big molecule, the chains can never move completely independently of each other, and the material can't form a new shape. Looked at in another way, those crosslinks tie the main chains in place. They may be able to move around some, but they can never get very far. If the amount of crosslinking is sufficient, they will always hold the material in the same basic shape.
Of course, just a little bit of crosslining may not have the same effect. You may have two or three chains tied together to form one big molecule, but they behave more like highly branched chains than like extended nets.
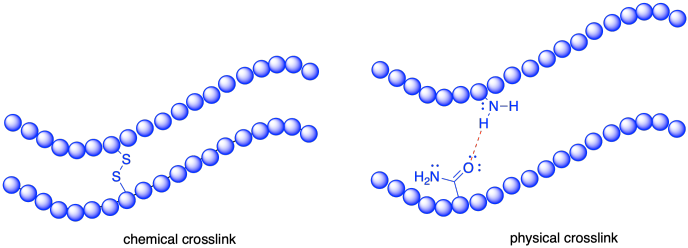
It's also worth noting that the term "crosslink" can actually mean different things when used in different ways. Sometimes, crosslinks refer to true covalent bonds connecting two chains together. These connections are called "chemical crosslinks"; the word chemical refers to the covalent bonds. Alternatively, chains can be connected to each other through strong intermolecular forces. That's not at all the same thing, of course, because these intermolecular forces can be overcome with sufficient energy, and so at some point the chains may no longer be tied together. These connections are called "physical crosslinks" to distinguish them from permanent bonds.
To use a biology analogy, sulfide bonds in proteins would be an example of chemical crosslinks; they hold the protein firmly in one shape, and a chemical reaction is required to break that connection. The ordinary hydrogen bonds that are so prevalent in the protein are physical crosslinks. Because they can be overcome by adding heat, proteins are very sensitive to temperature.
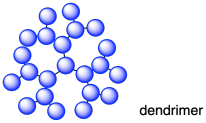
Dendrimers are another type of branched structure; the term comes from the Greek dendron or tree. Dendrimers differ from regular branched polymers in that they have a much more regular branching pattern. A dendrimer will actually grow outward from the center, branching out at regular intervals.
Many dendrimers are polyamides, although there are other types as well. Generally, at least one of the monomers is trifunctional, which introduces the branching in a predicatble way.
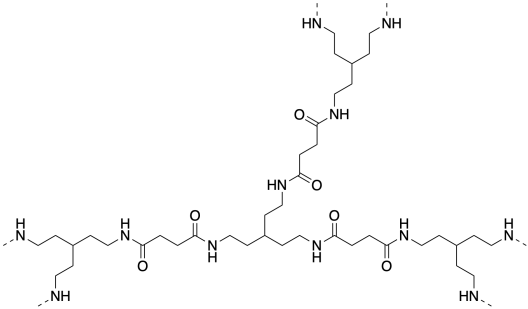
As dendrimers grow outward, layer by layer, they are often described in terms of generations. Suppose you start with a trifunctional monomer in the middle. That's generation 0. If the monomer is polymerized outward until there is another set of trifunctional monomers attached on the edge, we have a first generation dendrimer. If we keep going and add another layer of trifunctional monomers, we have a second generation dendrimer, and so on.
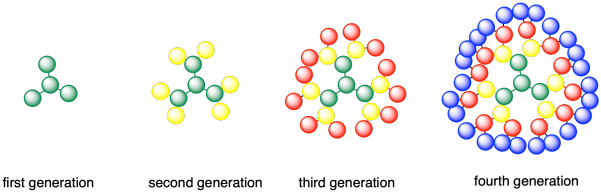
These dendrimers start to look circular on paper, like pancakes, but steric interactions between the groups forces things into three dimensions. As a result, dendrimers are roughly spherical in shape.
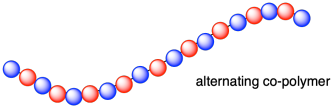
In some of the examples of polymers we have seen, the chain is actually composed of two different monomers. That's true in the case of polyamides such as nylon-6,6. In that example, the chain is composed of difunctional amines alternating with difunctional carboxyloids (such as carboxylic acids or acid chlorides). We can think of a polymer like that as being composed of two different monomers. Of course, because of their complementary reactivity they have to alternate: an amin and then a carboxyloid, to form an amide, and so on. We can think of these polymers as "co-polymers", meaning they are formed from more than one kind of monomer. We can go further and say that they are "alternating co-polymers" because the two different monomers alternate with each other along the chain.

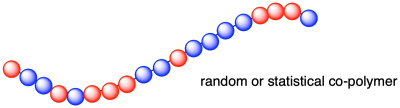
In some cases, there is no need for the two different monomers to alternate the way they do in nylon-6,6. If you take a mixture of alkenes that are capable of forming polymers and you polymerize them together, you may well get them randomly enchained into a growing polymer. This arrangement is called a "random co-polymer" or sometimes a "statistical co-polymer". For example, maybe you manage to get a random sequence of propene and vinyl chloride units along the polymer.
![]()
Notice that in the above example we still have the same zig-zag main chain in the polymer; all that changes between one monomer and the other is the group attached to that main chain. It is sometimes useful to think about this main chain or "backbone" separately from the attached or "pendant groups". In this case, we have what looks like a polyethylene backbone with pendant chlorides or methyls randomly attached.
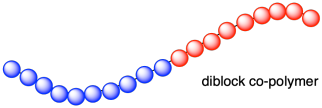
A random arrangement isn't the only possibility. Maybe all of the vinyl chlorides polymerized in a row, and then all of the propenes were incorporated after them. The result would be a diblock co-polymer; there is a solid block of poly(vinyl chloride) at one end of the chain and a solid block of polypropylene at the other end.
![]()
There are a couple of ways that could happen. Maybe you waited until all of the vinyl chlorides were enchained before adding any propene, so that all of the vinyl chlorides were enchained at the tail end and the propenes were added at the growing end later. Alternatively, maybe you added them all at once but the vinyl chlorides just underwent polymerization a whole lot faster than the propenes; the vinyl chlorides all became enchained before the propenes had a chance.
Those possibilities assume that the monomers are all enchained linearly. There are other possibilities. Maybe one set of monomers form the polymer backbone and the other set form pendant branches along the chain. That arrangement is called a "graft co-polymer", as though we have little apple trees grafted onto the trunk of another breed.
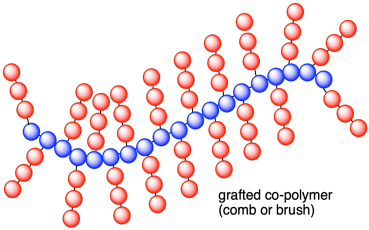
Problem MP8.1.
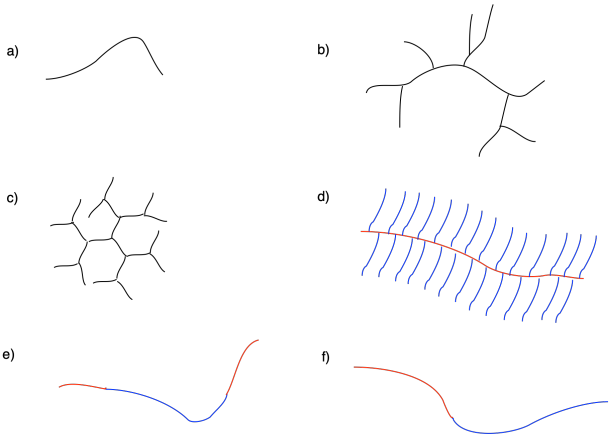
Describe the topology seen in each of the following cartoons.
Problem MP8.2.
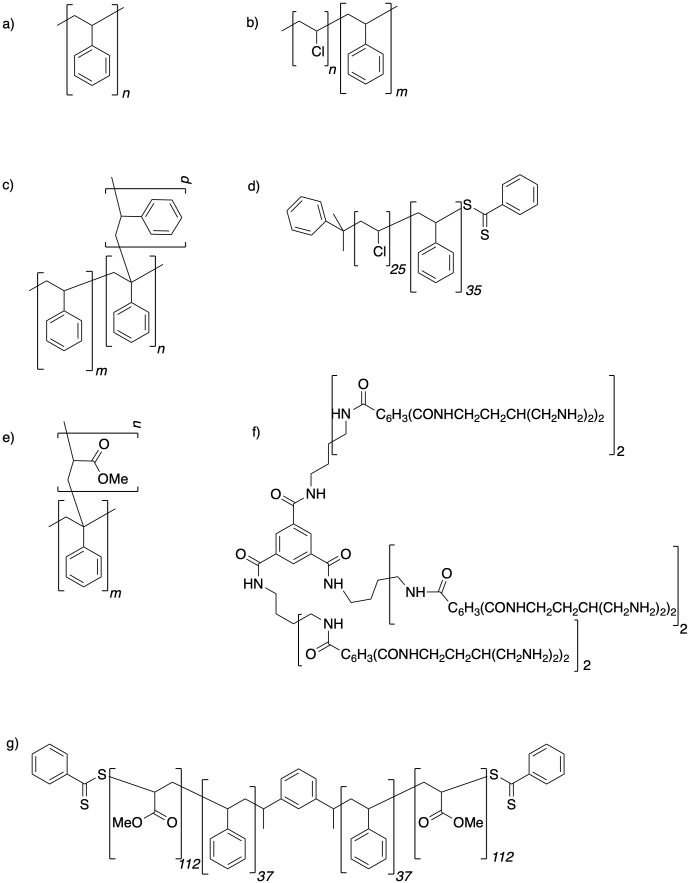
Describe the topology in each of the following structures.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Support for this project was provided by the Opens Textbooks Pilot Program of the U.S. Department of Education through a collaboration with the Libre Texts project at University of California, Davis.
Navigation:
Back to Structure & Reactivity